Adding a flux during the synthesis of oxyhydrides is a promising strategy to obtain a pure, homogenous product, reveal scientists from Tokyo Tech. An SrCl2 flux promoted the melting of a part of reactants and facilitated their diffusion of reactants, which proved to be the key to producing highly pure SrVO2.4H0.6 or Sr3V2O6.2H0.8 perovskite oxyhydrides in high-pressure and high-temperature reactions. These compounds have potential as catalysts and as electrode materials for lithium-ion batteries.
Perovskite oxyhydrides containing oxide (O2-) and hydride (H–) anions are promising compounds with applications in catalytic systems and batteries. Unfortunately, synthesizing oxyhydrides is usually quite challenging, mainly due to the highly reactive nature of H– anions.
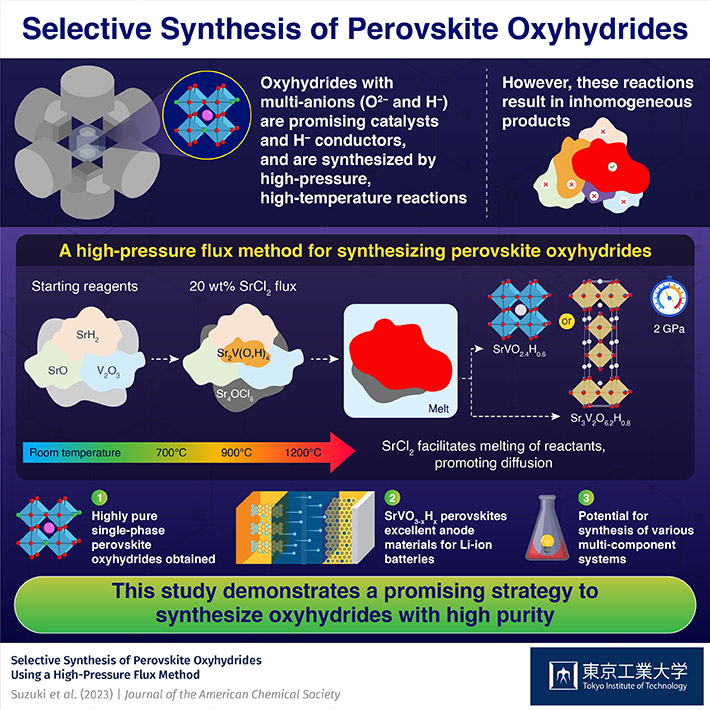
It was known that high-pressure and high-temperature reactions are effective to synthesize oxyhydrides. For example, Sr2VO4-xHx perovskite can be synthesized directly from oxide and hydride precursors in high-pressure and high-temperature reactions. A key advantage of these reactions is that the H– content in the final product can be tuned by adjusting the composition and ratio of the precursors. This essentially means that the electronic and magnetic properties of the product are also customizable. Unlike Sr2VO4-xHx, synthesizing SrVO3-xHx has proven much more difficult, since the necessary high-pressure and high-temperature reactions lead to the formation of several impurities and inhomogeneous products, mainly due to insufficient diffusion of the solid components.
In a recent study published in , a research team led by Associate Professor Takafumi Yamamoto from the Institute of Innovative Research at Tokyo Institute of Technology (Tokyo Tech) found a solution to this problem. They developed a novel approach to synthesize highly pure SrVO2.4H0.6 and Sr3V2O6.2H0.8, two new perovskite oxyhydrides. This study was conducted as part of a collaborative research project with the ³Ô¹ÏÍøÕ¾ Institutes for Quantum Science and Technology, Japan.
The researchers started with SrO, SrH2, and V2O3, and added SrCl2 to these reactants. They observed the differences in the composition of samples prepared under different conditions using a technique called in-situ synchrotron X-ray diffraction, shedding light on the role of SrCl2 in the reaction. It acted as a flux at a high temperature of 1,200 ℃ and a high pressure of 2 GPa, facilitating the melting and dissolution of a part of reactants, thus promoting diffusion. Consequently, the researchers managed to suppress the development of inhomogeneous products that typically appear due to insufficient diffusion, obtaining highly pure SrVO2.4H0.6 or Sr3V2O6.2H0.8 perovskite oxyhydrides.
Additionally, the team analyzed the electrochemical properties of the prepared perovskite oxyhydrides as an electrode material. “With low working potential, excellent reversibility, and high-rate characteristics, SrVO3-xHx could be suitable as a negative electrode for lithium-ion batteries, a first for oxyhydrides,” highlights Dr. Yamamoto.
Overall, using a flux to boost the desired reaction pathways in high-pressure and high-temperature reactions could be a powerful strategy to unlock a plethora of new compounds beyond perovskite oxyhydrides. Dr. Yamamoto remarks: “The proposed synthesis approach would also be effective in the synthesis of various types of multi-component systems.”
Let us hope that these findings lead to new breakthroughs in energy storage and other areas of applied chemistry!
Reference
Authors : | Jinya Suzuki1, Hiroyasu Okochi1, Naoki Matsui2, Teppei Nagase1, Haruki Tochizawa1, Hiroki Sahara1, Takumi Nishikubo3,1, Yuki Sakai3,1, Takuya Ohmi1, Zhao Pan1, Takashi Saito4, Hiroyuki Saitoh5, Atsunori Ikezawa6, Hajime Arai6, Ryoji Kanno2, Takafumi Yamamoto1, and Masaki Azuma1,3,7 |
Title : | Selective Synthesis of Perovskite Oxyhydrides Using a High-Pressure Flux Method |
Journal : | Journal of American Chemical Society |
DOI : | |
Affiliations : | 1Laboratory for Materials and Structures, Institute of Innovative Research, Tokyo Institute of Technology 2Research Center for All-Solid-State Battery, Institute of Innovation Research, Tokyo Institute of Technology 3Kanagawa Institute of Industrial Science and Technology 4Institute of Materials Structure Science, High Energy Accelerator Research Organization (KEK) 5³Ô¹ÏÍøÕ¾ Institutes for Quantum Science and Technology 6School of Materials Science and Technology, Tokyo Institute of Technology 7Living Systems Materialogy (LiSM) Research Group, International Research Frontiers Initiative (IRFI), Tokyo Institute of Technology |







