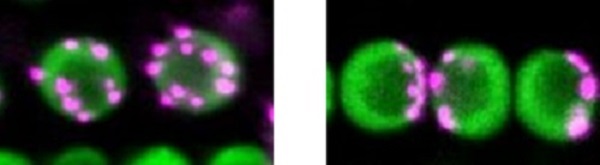
non-Rabl and Rabl configuration
The left panel shows non-Rabl configuration. Centromeres in magenta dispersed in nuclei in green. The right panel shows Rabl configuration. Centromeres unevenly distributed in nuclei. ©2022 Sachihiro Matsunaga, The University of Tokyo
Since the 1800s, scientists have noted configuration of centromeres, a special chromosomal region that is vital for cell division, in the nucleus. Up until this point, however, the determining mechanisms and the biological significance of centromere distribution were poorly understood.
A team led by researchers from the University of Tokyo and their collaborators recently proposed a two-step regulatory mechanism that shapes centromere distribution. Their findings also suggest that centromere configuration in the nucleus plays a role in maintaining genome integrity. The results were published in Nature Plants.
During the process of cell division, special chromosomal domains called centromeres are pulled to the opposite ends of the cell. After cell division is completed and the cell nucleus is constructed, centromeres spatially distributed in the nucleus. If the distribution of centromeres pulled to the two poles remains unchanged, the cell nucleus will have centromeres grouped at only one side of the nucleus. This uneven distribution of centromeres is called Rabl configuration, after the 19th-century cytologist Carl Rabl. Some species’ nuclei show a dispersed distribution of centromeres instead, which is known as non-Rabl configuration.
“The biological function and molecular mechanism of the Rabl or non-Rabl configuration has been a mystery across the centuries,” said corresponding author Sachihiro Matsunaga, professor at the University of Tokyo’s Graduate School of Frontier Sciences. “We successfully revealed the molecular mechanism to construct the non-Rabl configuration.”
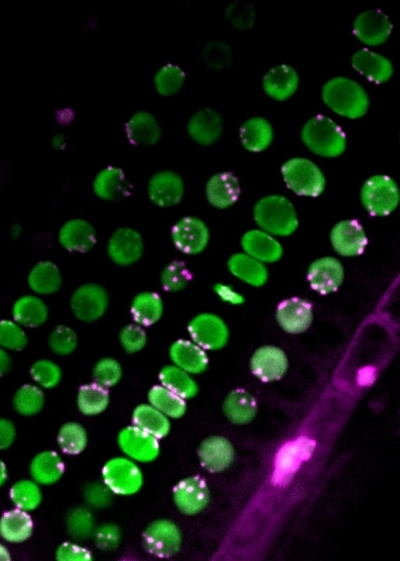
Centromere distribution in the root
The uneven distribution of centromeres (magenta) in nuclei (green). ©2022 Sachihiro Matsunaga, The University of Tokyo
The researchers studied the plant Arabidopsis thaliana, known also as thale cress and a specimen that is known to have non-Rabl configuration, and its mutant form that had a Rabl configuration. Through their work, they found that protein complexes known as condensin II (CII) and protein complexes known as the linker of nucleoskeleton and cotoskeleton (LINC) complex work together to determine centromere distribution during cell division.
“The centromere distribution for non-Rabl configuration is regulated independently by the CII– LINC complex and a nuclear lamina protein known as CROWDED NUCLEI (CRWN),” Matsunaga said.
The first step of the two-step regulatory mechanism of centromere distribution that the researchers uncovered was that the CII-LINC complex mediates the scattering of centromeres from late anaphase to telophase — two phases at the end of cell division. The second step of the process is that the CRWNs stabilize the scattered centromeres on nuclear lamina within the nucleus.
Next, to explore the biological significance, the researchers analyzed the gene expression in A. thaliana and in its Rabl-structure mutant. Because a change in the spatial arrangement of centromeres also changes the spatial arrangement of genes, the researchers expected to find differences in gene expression, but this hypothesis proved to be incorrect. However, when DNA damage stress was applied, the mutant grew organs at a slower rate than the normal plant.
“This suggests that precise control of centromere spatial arrangement is required for organ growth in response to DNA damage stress, and there is no difference in tolerance to DNA damage stress between organisms with the non-Rabl and Rabl,” Matsunaga said. “This suggests that the appropriate spatial arrangement of DNA in the nucleus regardless of Rabl configuration is important for stress response.”
According to Matsunaga, the next steps are to identify the power source that changes the spatial arrangement of specific DNA regions and the mechanism that recognizes specific DNA.
“Such findings will lead to the development of technology for artificially arranging DNA in the cell nucleus in an appropriate spatial arrangement,” he said. “It is expected that this technology will make it possible to create stress-resistant organisms, as well as to impart new properties and functions by altering the spatial arrangement of DNA rather than editing its nucleotide sequence.”








