Scientists are urgently searching for clean fuel sources – such as hydrogen – to move towards carbon neutrality. A breakthrough for improving the efficiency of the photocatalytic reaction that splits water into hydrogen has been made by a team of researchers from Tohoku University, Tokyo University of Science and Mitsubishi Materials Corporation.
“Water-splitting photocatalysts can produce hydrogen (H2) from only sunlight and water,” explains Professor Yuichi Negishi, the lead researcher of this project (Tohoku University), “However, the process hasn’t been optimized sufficiently for practical applications. If we can improve the activity, hydrogen can be harnessed for the realization of a next-generation energy society.”
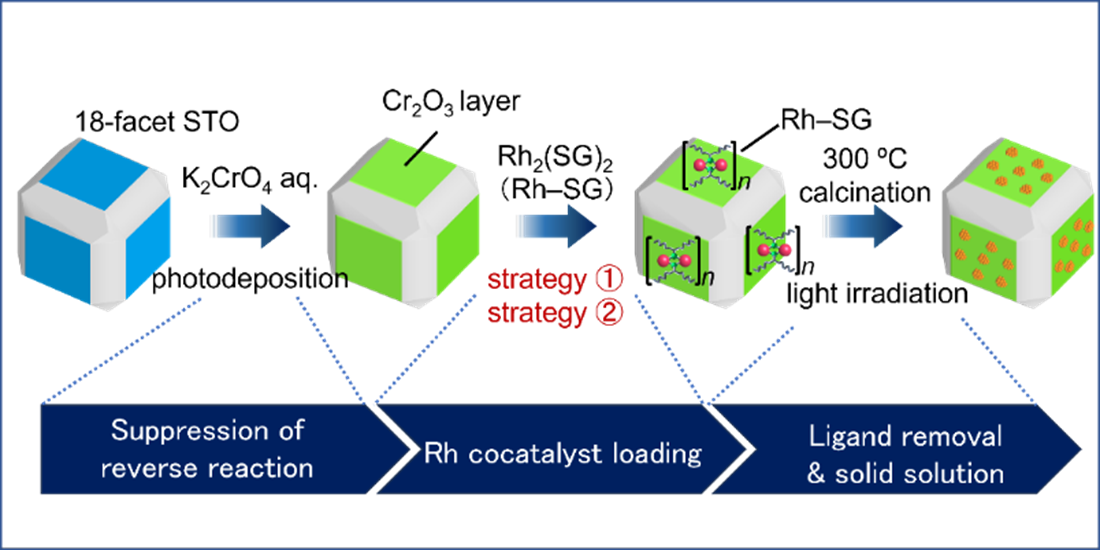
The research team established a novel method that uses ultrafine rhodium (Rh)-chromium (Cr) mixed-oxide (Rh2-xCrxO3) cocatalysts (the actual reaction site and a key component to stop H2 reforming with oxygen to make water again) with a particle size of about 1 nm. Then, they are loaded crystal facet-selectively onto a photocatalyst (uses sunlight and water to speed up reactions). Previous studies have not been able to accomplish these two feats in a single reaction: a tiny cocatalyst that can also be placed on specific regions of the photocatalyst.
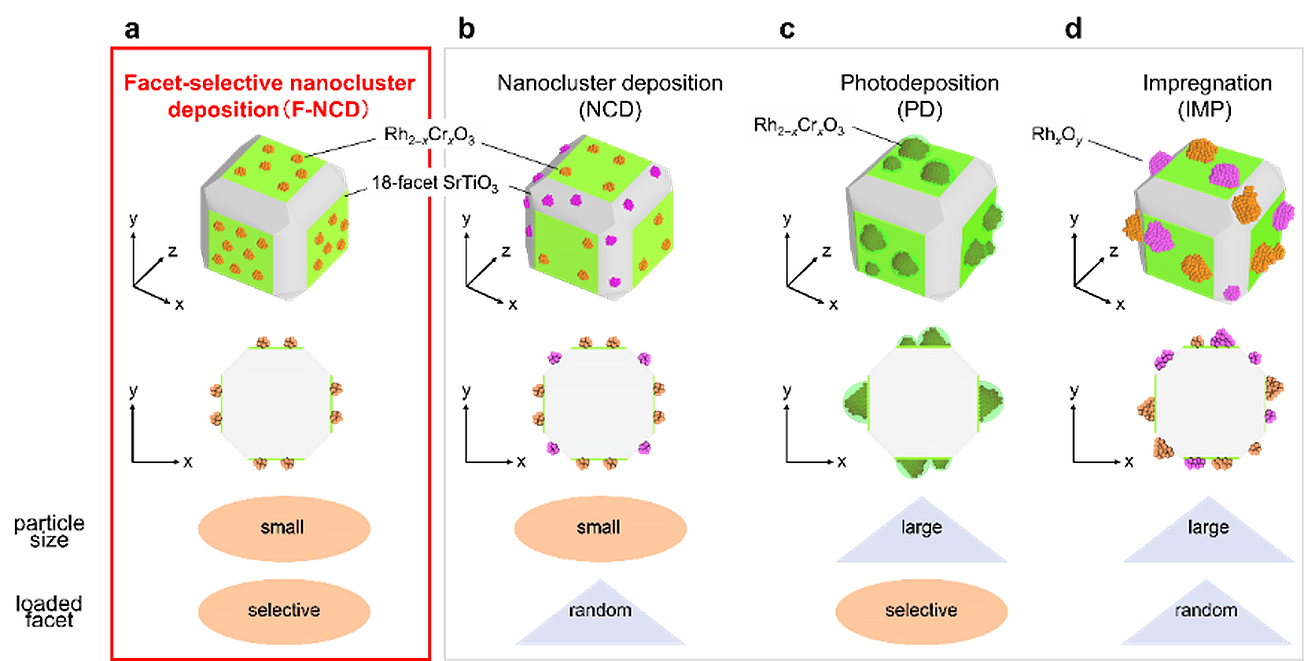
A smaller particle size is important because then the activity per amount of cocatalyst loaded is greatly enhanced due to the increase in specific surface area of the cocatalyst. Facet-selective loading is also important, because otherwise, randomly placed cocatalysts may end up on crystal facets where the desired reaction does not occur.
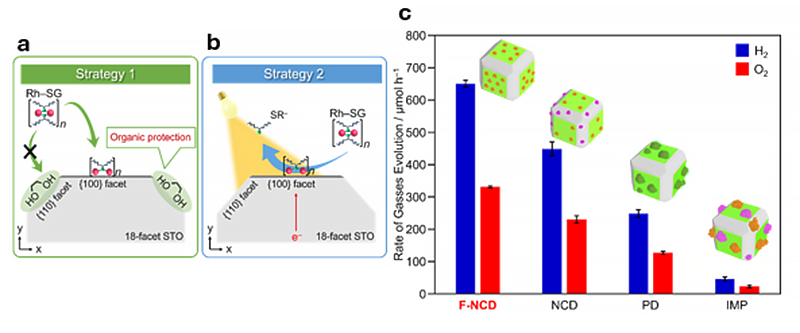
The particle size, loading position, and electronic state of the cocatalyst in the photocatalyst prepared by the F-NCD method (Rh2-xCrxO3/18-STO (F-NCD)) were compared with those prepared by the conventional method. Overall, photocatalysts prepared by the new method achieved 2.6 times higher water-splitting photocatalytic activity. The resulting photocatalyst exhibits the highest apparent quantum yield achieved to date for strontium titanate.
This remarkable method has improved our ability to generate hydrogen without harmful byproducts such as carbon dioxide. This may allow us to harness hydrogen as a more abundant, green energy source so we can all breathe a little easier.
The results of this research have been published in the Journal of the American Chemical Society on September 23, 2024.
- Publication Details:
Title: Ultrafine Rhodium-Chromium Mixed-Oxide Cocatalyst with Facet-Selective Loading for Excellent Photocatalytic Water Splitting
Authors: Daisuke Hirayama, Tokuhisa Kawawaki*, Sota Oguchi, Mai Kogano, Naoaki Kon, Tomohiro Yasuda, Akihiro Higami, Yuichi Negishi*
Journal: Journal of the American Chemical Society; Design
DOI:








