An advanced open-source digital platform developed by QUT researchers is being used to build a patient registry aimed at improving outcomes and health service provision for children and adults around the world living with the rare disease Angelman syndrome.
Known as the Trial Ready Registry Framework (TRFF), the platform supports the , a patient and caregiver-led resource initiated by the .
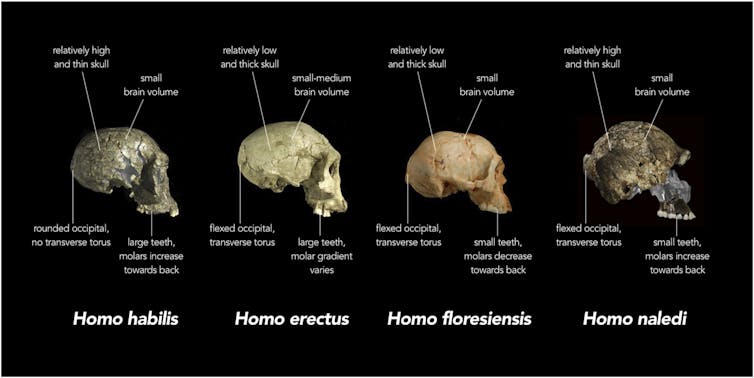
Angelman syndrome (AS) is a neurodevelopmental disorder with no cure. Worldwide it affects about 1 in 15,000 children and causes intellectual disability, epilepsy, and problems with speech, movement and balance. A common characteristic is a happy and easily excitable personality.
QUT Director of and TRRF project lead, Professor Matt Bellgard, said the registry aims to be the largest and most comprehensive source of information about people living with AS globally to:
- raise awareness and understanding of the condition
- provide valuable information to health professionals and service providers
- enable researchers conducting clinical therapy trials and other studies on AS to recruit patients more easily and evaluate treatments.

The global AS registry was launched four years ago with an earlier version of the digital platform. Data has now been migrated to the upgraded platform, which provides analytical capabilities and enhanced functionality for clinician and researcher access, Professor Bellgard said.
“Previously it was about simply capturing data, but now we’re enabling purposeful applications of that data,” he said.
“The platform provides real-time analytics and supports the embedding of data and outcomes of clinical trials into AS therapies that are being conducted.
“Each patient’s journey is different, how they are affected by the condition is unique to them, and they often see multiple medical specialists for various issues so may be in multiple digital medical systems. Having a central point for data, aggregating it in a sophisticated way, housing it securely, with privacy and governance protocols in place, means providing a more detailed and longitudinal picture for clinicians and researchers.”
FAST Australia will continue to promote the registry to encourage more families and caregivers of children and adults with AS to participate.
“We already have about 1400 people from 64 countries on the registry,” said FAST Australia chair Meagan Cross, whose youngest daughter Molly was diagnosed with AS in 2008 when she was aged about 13 months.

“It’s estimated there are about 500,000 people with AS worldwide, so we have a lot of work to do still. We believe people will find the new platform more user-friendly and will be able to engage with it more.
“It’s important that we not only collect data but that it can be used to help improve outcomes for people living with AS, and advance clinical trials and research towards a cure.
“One of the goals with the registry is to be able to understand what the various characteristics of AS mean for individuals and what therapies may have been or may be effective.
“At the moment we use social media to share information. Someone might ask ‘How many people take this medication?’. Using the data, we’d be able to say, ‘According to the registry, 80 per cent of patients take that medication’.
“We’ll be able to learn a lot, and we hope to be able to encourage medical professionals to seek out information to aid in providing care. It would have been a good resource to have been available when my daughter was diagnosed. My doctor had to Google AS while I was sitting in his office.
“We’re looking forward to working with the team of experts in eResearch and digital health systems at QUT to build on this resource.”
Establishment of the Global Angelman Syndrome Registry using TRRF was funded by MTPConnect (Medical Technologies and Pharmaceuticals Growth Centre) and FAST Australia.
Professor Bellgard said the framework will also be used to establish patient and clinician registries for other rare diseases.
“This framework is ideal for the rare disease world,” he said. “There are as many as 7000 unique medical conditions that fall under the umbrella of rare disease.
“Sometimes only a handful of people in various parts of the world might have a particular condition and there is no central coordinating rare disease data resource to assist those people and inform healthcare professionals and researchers. Many people can only share information informally through networks they have set up either through clinical networks or for families through social media.”