
Imagine a self-repairing rubber, or super-adhesive made entirely from waste materials.
It sounds like science fiction or a scene from The Terminator, but researchers have discovered a new kind of rubber and catalyst that together can be used with low energy consumption to make flexible, repairable, sustainable objects – including car tyres.
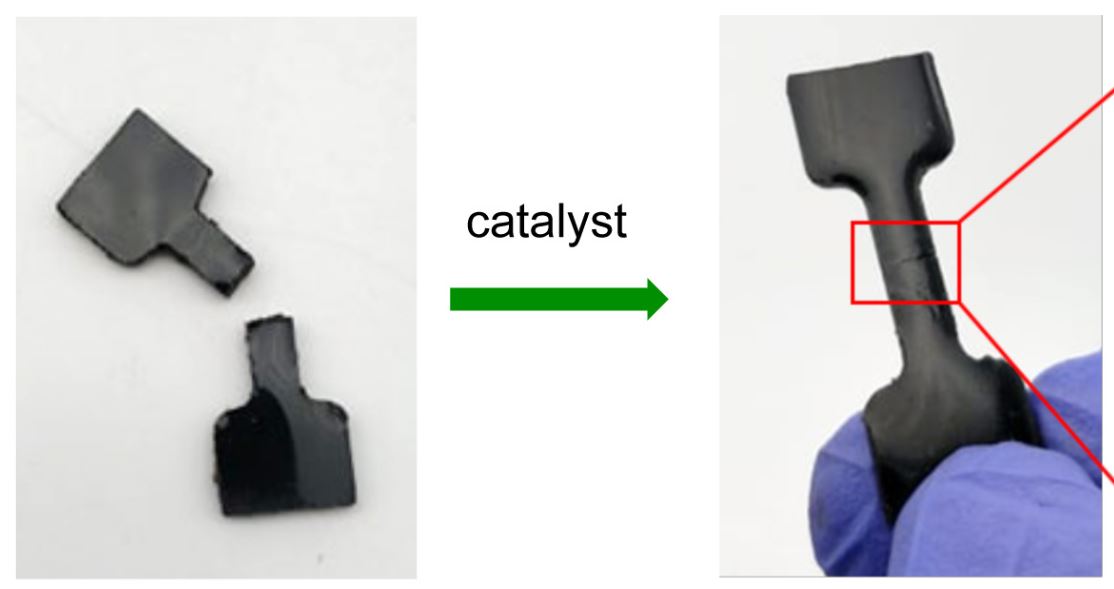
The new rubber material, made from cheap and plentiful industrial waste products sulfur, canola cooking oil and dicyclopentadiene (DCPD) from petroleum refining, can be completely repaired and returned to its original strength in minutes – even at room temperature – with an amine catalyst.
The new type of rubber can be seamlessly repaired if damaged and can also be recycled, says research leader , whose team’s in leading international journal Chemical Science.
The amine catalyst used to trigger the reaction that causes the rubber to self-repair occurs within minutes in some cases and it is all done at room temperature, scientists say.
“This study reveals a new concept in the repair, adhesion and recycling of sustainable rubber,” says Associate Professor Chalker, adding too many plastics, rubbers and ceramics are not recyclable.
Each year in Australia, the equivalent of 48 million tyres reach the end of their life, only 16% of these are domestically recycled. Around two-thirds of used tyres in Australia end up in landfill, are stockpiled, illegally dumped or have an unknown fate.
This represents both a waste of resources and creates health and environmental issues. Each passenger car tyre contains approximately 1.5kg of steel, 0.5kg of textiles and 7 kg of rubber. – Source: Planet Ark
“It is exciting to see how the underlying chemistry of these materials has such wide potential in recycling, next-generation adhesives, and additive manufacturing,” Associate Professor Chalker says.
Researchers from the Chalker Lab at the Flinders University Institute for Nanoscale Science and Technology, with University of Liverpool and University of Western Australia colleagues, say the new rubber can be used as a “latent adhesive”.
“The rubber bonds to itself when the amine catalyst is applied to the surface. The adhesion is stronger than many commercial glues,” says University of Liverpool researcher Dr Tom Hasell.
“The polymer is also resistant to water and corrosion.”
Rubber bricks made out of this polymer can be chemically joined by applying the catalyst.
“In some cases, the amine catalyst causes the rubber to bond in just minutes, and it can be done at room temperature,” explains Flinders University lead author Sam Tonkin.
“The rubber can also be used as a latent adhesive, where it bonds to the surface of another piece of rubber when the amine catalyst is applied.
“Basically the rubber is not ‘sticky’ until the catalyst is applied.”
In addition to the highly useful practical applications, the new paper gives detailed fundamental studies on the mechanisms of the rubber repair.
‘‘ (May 2020) by SJ Tonkin, CT Gibson, JA Campbell, DA Lewis, A Karton, T Hasell and JM Chalker in Chemical Science (The Royal Society of Chemistry) DOI: 10.1039/D0SC00855A https://doi.org/10.1039/D0SC00855A
Funding acknowledgements – Australian Research Council (DP200100090 and FT170100373), AMP Foundation, Flinders University Impact Seed Funding for Early Career Researchers, the Royal Society, Ferry Trust, the South Australian node of Microscopy Australia and the Australian ³Ô¹ÏÍøÕ¾ Fabrication Faculty and ³Ô¹ÏÍøÕ¾ Computational Infrastructure.








