Scientists at Tokyo Institute of Technology (Tokyo Tech) have shown that single platinum atoms trapped in C12A7 crystals act as a stable and effective catalyst for the hydrogenation of nitroarenes, an essential process in the production of many kinds of fine chemicals. Their approach could become a versatile route for developing other single-atom catalysts for wide-ranging industrial applications.
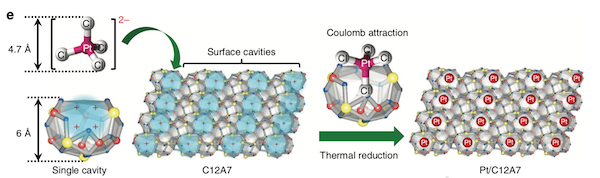
Figure 1. Single platinum atoms stabilized in C12A7 crystals
Interaction between negatively charged ions and positively charged surface cavities of C12A7 for effective stabilization of single platinum atoms.
Single-atom catalysts (SACs) are on the way to becoming dream catalysts ― ones that exhibit superb performance based on optimized usage of metal atoms. Many research teams around the world have been working to advance the scalable development of SACs since they were first proposed by Tao Zhang and colleagues in China and the US in 2011.
Now, in a proof-of-concept study that throws the door wide open to developing a new range of SACs, researchers at Tokyo Tech have designed and tested a catalyst composed of single platinum atoms trapped in C12A7, a nanoporous crystal widely used in the production of aluminous cement.
The inner structure of C12A7 crystals is “just the right size” for trapping single metal atoms, the researchers say in their paper published in Nature Communications.
“Our approach is rather like a ‘diamond-in-a-ring’ strategy, where the surface cavity of C12A7 can be regarded as a ring, and the single platinum atom is fixed on the ring as a diamond,” says first author Tian-Nan Ye at Tokyo Tech’s Materials Research Center for Element Strategy.
Ye explains that C12A7 has a positively charged framework structure composed of twelve sub-nanometer-sized cages, each with an inner diameter of around 0.4 nanometers ― a suitable size for capturing individual metal atoms. Each cage has a positive charge of +1/3, and the surface cavities have an open ‘mouth’ that can trap single metal atoms through electronic interaction.
The catalyst has been demonstrated to be highly stable and active toward the selective hydrogenation of nitroarenes, an important process often used in the dye and polymer industries. It has a higher turnover frequency (up to 25772 per hour) than that of platinum-based catalysts unsupported by C12A7. Remarkably, the new catalyst even works at temperatures of up to 600°C.
Based on these promising results, the researchers investigated whether the trapping effect might work using other metals. As they predicted, C12A7 was also capable of capturing single atoms of ruthenium and rhodium, indicating that their strategy would be applicable to various transition metals.
“Our findings open countless doors to developing new kinds of SACs for different catalytic processes,” says Ye. Due to its exceptionally high thermal stability, the C12A7 support would be able to withstand harsher conditions involved in other industrially important processes such as ammonia synthesis and CO2 reduction.
Ye points out that the development of SACs cannot be separated from the exploration of new materials. This is a key reason why Professor Hideo Hosono’s group at Tokyo Tech is uniquely positioned to be a pioneer in SAC research, he says, building on a series of achievements including the development of , an , and the first .