Key Points
Research brings insights into the complex immune response that occurs in the brain
Experiments revealed that low dose radiation reduces the activation state of the brain’s innate immune system and may reduce ‘neuroinflammation’
Health researchers at ANSTO have conducted experiments using the TSPO knockout model to assess the brain’s adaptive response to radiation in low doses
Researchers at ANSTO have discovered that low dose radiation causes a markedly different response by the brain’s own immune cells, the microglia than would have been predicted from high dose radiation experiments and clinical observations in radiation therapy.
While high dose radiation activates microglia and leads to their transformation into macrophages (cells that remove injured tissue), low dose radiation causes not merely less activation of microglia but quite the opposite: it actually reduces the baseline activation state of the brain’s innate immune system.
This has led to speculation that the observed reduction in the brain’s pro-inflammatory immune response might be an evolutionary-evolved mechanism of protection and that, in disease conditions with a detrimental over-active inflammatory response, low dose radiation may dampen an over-active immune response.
The ANSTO research team led by A/Prof Guo-Jun Liu, Biology Group Leader, Human Health has been studying the effects of low dose radiation both in cell culture and laboratory animals.
Low dose irradiation is usually classified as radiation being of 100 milligray or lower, although it does not account for differences between species or the cumulative dose. Milligray is the unit of measurement for the absorbed dose of radiation.
The has been published in Frontiers in Cell and Developmental Biology and further described in a published in Cells.
The team at ANSTO included lead author Dr Calina Betlazar, a PhD student at the University of Sydney and an AINSE Post Graduate Research Award recipient, Dr Ryan Middleton, Alexandra Boyd, Nicholas Howell, Prof Richard Banati, Ben Storer, Sarah Byrne, Emma Davis and Dr Justin Davies.
Activated microglia play an important role in inflammation or injury of the brain, broadly captured under the term ‘neuroinflammation’.
‘Neuroinflammation’ has been described in both acute and chronic diseases of the nervous system, such as brain trauma, stroke, cancer, Alzheimer’s disease and Parkinson’s disease but also as part of brain development and plasticity. It is likely that ‘neuroinflammation’ plays a role in injury as well as repair.
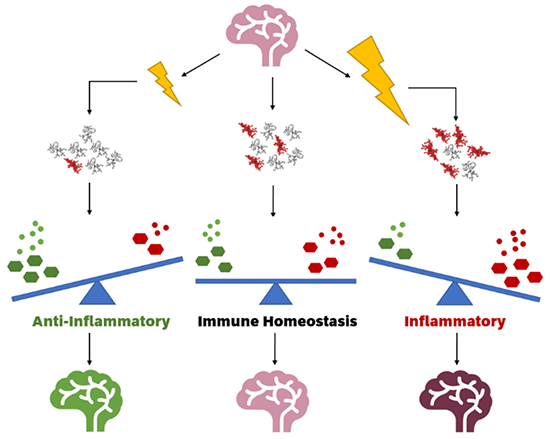
Ionising radiation modulates inflammatory response in a healthy brain by altering microglial functional states. Low dose ionising radiation (left) may reduce the number of activated microglia, increasing antioxidants and anti-inflammatory cytokines and thus showing an adaptive effect when compared to a control brain (middle). High dose ionising radiation (right) increases the number of activated microglia, which increases oxidants and pro-inflammatory cytokines, creating a neuroinflammatory state. (Boyd et al., Cells, 2021)
Microglia activation by internal and external stressors modulates ‘neuroinflammation’ through the release of pro-inflammatory molecules and other biomolecules.
This research builds on the earlier work of the ANSTO team, who created a mitochondrial translocator protein (TSPO) knockout mouse model.
The TSPO is closely linked to the energy production of the cell and responds to a very broad range of stressors, including behavioural, hormonal, metabolic dysregulation, as well as immune responses and subtle tissue injuries that are often not detected by conventional diagnostic imaging or assessments of microscopic biological samples.
The measurement of TSPO expression levels is one of the most useful and highly sensitive markers of stress response. For this reason, TSPO was chosen as one marker to assess radiation effects at doses so low that generally there is no readily identifiable tissue damage.
The research published in Frontiers in Cell and Developmental Biology found TSPO levels in the brain decrease following low dose irradiation, leading to a reduction in the activation of microglia, thereby a reduction in ‘neuroinflammation’.
“While is there is not nearly enough evidence to suggest any clinically beneficial effects of low dose radiation as such, it is intriguing to observe the non-linearity of biological responses to low dose radiation which seems not only less but different. Such non-linear behaviour is known from toxic or bioactive compounds given at low doses.
“In the context of radiation, it is worth noting that the background levels of radiation on Earth have been significantly higher than today and life evolved under these conditions, adapting to these through repair and tolerance mechanisms.
“The vision of space travel has rekindled the interest in exploring these repair and tolerance mechanisms further,” said Prof Richard Banati of ANSTO and Chair of Medical Radiation, University of Sydney.
DOI: