Researchers make the proton pump of the respiratory chain work in an artificial polymer membrane
Researchers from the Max Planck Institute for Dynamics of Complex Technical Systems in Magdeburg, the Max Planck Institute of Colloids and Interfaces in Potsdam, and the University of Halle are one step closer towards a synthetically constructed cell. They have used an enzyme found in bacteria to assemble one crucial part of the respiratory chain – essential for the energy metabolism in many cells – and made it functional in an artificial polymer membrane.
Creating artificial cells is one of the great visions in both biology and engineering. Some of the ambitious visionaries radically rebuild cells that already exist in nature. Others – like the Max Planck researchers – take an even rockier road. “We want to construct a new cell from scratch by gradually combining individual components into a living system with a metabolism”, says Ivan Ivanov, a scientist from the working group of Kai Sundmacher, Director at the Max Planck Institute in Magdeburg.
In a recent study, the researchers looked for an artificial polymer that has the properties of a cell membrane and could also play its role in energy metabolism. Natural cell membranes, which consist of phospholipids, separate the cell interior from the environment. They have both hydrophilic and lipophilic properties and are the stage for essential biochemical reactions that serve to produce energy for the cell, among other things. “Inspired by the natural processes from the energy metabolism of living organisms, we design customized artificial energy organelles from biological and chemical building blocks that convert light or chemical energy into ATP”, explains Tanja Vidaković-Koch from the Max Planck Institute for Dynamics of Complex Technical Systems. Nearly all chemical reactions in the cell are fuelled by ATP.
Proton pump in an artificial membrane
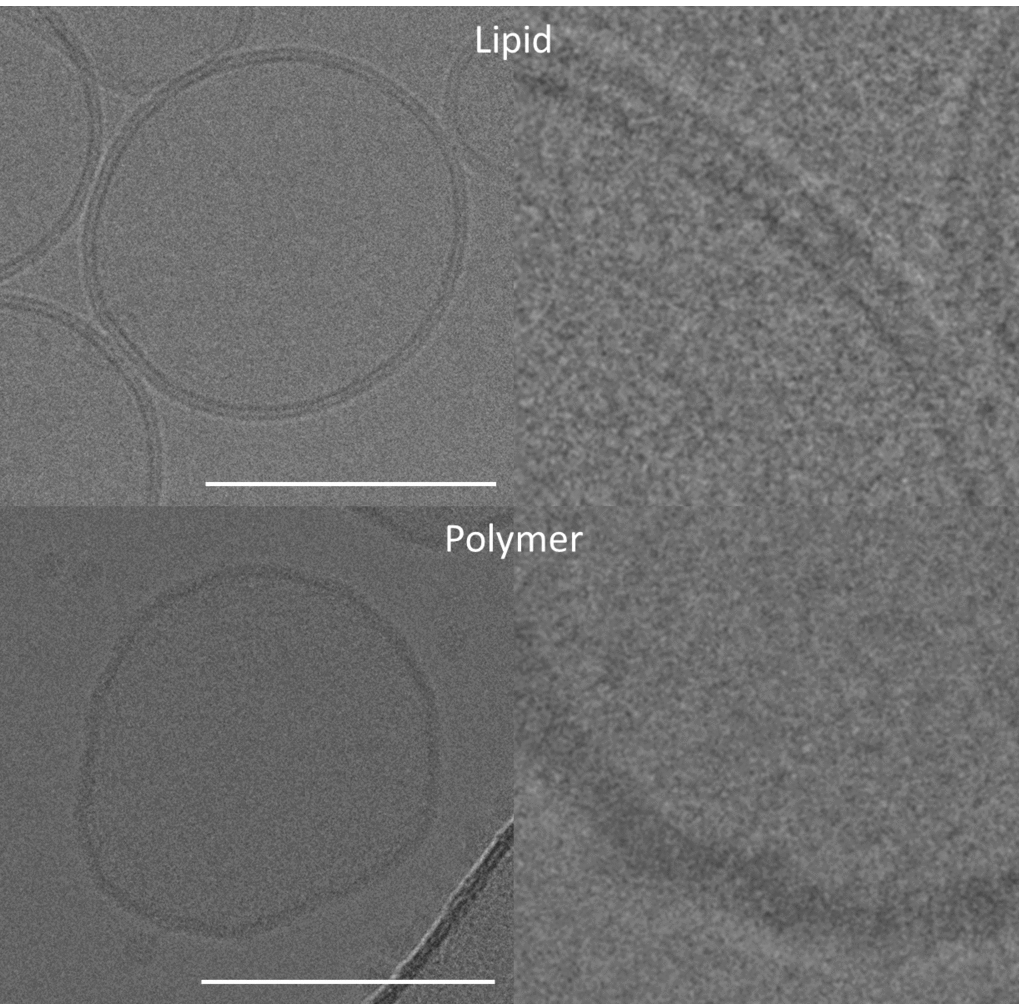
The researchers have now found a commercially available polymer (the surfactant PDMS-g-PEO) that acts as a membrane in place of the natural phospholipids and can thus form vesicles. Such vesicles “are a useful model for the construction of artificial organelles and cells”, explains Rumiana Dimova, a specialist for biomembranes at the Max Planck Institute of Colloids and Interfaces. A major obstacle has been incorporating functional proteins – including those involved in energy metabolism – into polymer membranes.
The team of Max Planck scientists has now succeeded in integrating the proton pump bo3 oxidase into the synthetic membrane. The enzyme belongs to the respiratory chain of many bacteria “and also functions quite well in the polymer membrane – even slightly better than in the natural lipid membranes”, says Nika Marušič, co-author of the study.
The oxidase reduces oxygen also in the artificial membrane and thus constitutes the final step of cellular respiration. As the researchers have shown, it pumps protons into the interior of the vesicle, thereby creating a prerequisite for the production of ATP.
Impermeable to protons
The artificial membrane is also nearly impermeable to protons, yet sufficiently fluid and highly stable (much more stable than its natural counterpart) against harmful oxygen radicals. The bending rigidity of the polymer membrane is also similar to that of a natural membrane. This is important because living cells are constantly deforming. The bending modulus must therefore not be too low so that the cells can maintain their shape. However, it also should not be too high either. Otherwise, the function of complex membrane proteins will be compromised.
To put it simply: the chemistry of the polymer offers excellent conditions for energy metabolism in an artificial mitochondrion. According to Ivanov, there are still some obstacles though: “It is still unclear how this polymer membrane could replicate”. This would certainly be necessary for an artificial cell to be able to multiply. The scientists thus still have a great deal of work ahead of them.

Marušič et al, PNAS 2020






