In a recent study published in the multidisciplinary academic journal Science Bulletin, a semi-monthly high-caliber peer-reviewed research outlet covering a broad range of natural sciences and high-tech fields, researchers from the Nano Life Science Institute (WPI-NanoLSI) at Kanazawa University utilized hopping probe scanning ion conductance microscopy (HPICM) (※1) and highly sensitive platinum-functionalized nanoelectrodes to conduct an in-depth investigation of the dynamic response of individual living colorectal cancer Caco-2 cells to changes in intracellular and extracellular hydrogen peroxide (H2O2) gradients, specifically focusing on eustress, at the single-cell level and in real-time. Their findings hold promise for innovative therapies against cancer and H2O2-related inflammatory diseases.
————————————————————————————————————————————————————————————————
Reactive Oxygen Species (ROS) production plays a significant role in various diseases, including atherosclerosis, chronic obstructive pulmonary disease, and cancer. Despite promising preclinical antioxidant therapies, clinical trials have yielded unsatisfactory results. Hydrogen peroxide (H2O2), a stable ROS derivative, acts as a crucial participant in physiological signaling by serving as a eustressor. The concentration of H2O2 in the tumor microenvironment is closely linked to the occurrence and progression of colorectal cancer. A deeper understanding of how H2O2 functions and its specific efficacy in individual cancer cells may pave the way for more effective ROS-related treatments.
The authors utilized hopping probe scanning ion conductivity microscopy (HPICM) alongside Pt-functionalized nanoelectrodes to quantitatively assess the dynamic response in cell morphology, cell mechanical properties, and extracellular to intracellular eustress H2O2 gradients of individual colorectal cancer Caco-2 cells under H2O2 eustress. The investigation revealed that exposure to 0.1 or 1 mmol/L H2O2 eustress increased the extracellular to intracellular H2O2 gradient from 0.3 to 1.91 or 3.04, respectively. Additionally, the study found that F-actin-dependent cell stiffness increased under eustress of 0.1 mmol H2O2 but decreased under eustress of 1 mmol H2O2. Furthermore, eustress-induced cell stiffness was positively regulated by AKT activation and negatively influenced the expression of the H2O2-scavenging enzyme GPX2, ultimately maintaining relatively stable cellular H2O2 levels.
This study suggests that the eustress induced by low levels of H2O2 may contribute to the failure of some H2O2 targeted therapies. The results uncover a novel interplay between cellular physical properties and biochemical signaling in cancer cells’ antioxidant defense, shedding light on the utilization of H2O2 eustress for survival at the single-cell level. This understanding is critical for comprehending cancer development and other H2O2-related inflammatory diseases. Inhibiting GPX under H2O2 eustress resulted in cytotoxicity, suggesting a potential enhancement for colon cancer treatment. These findings hold promise for the development of innovative therapies targeting cancer and H2O2-related inflammatory diseases.
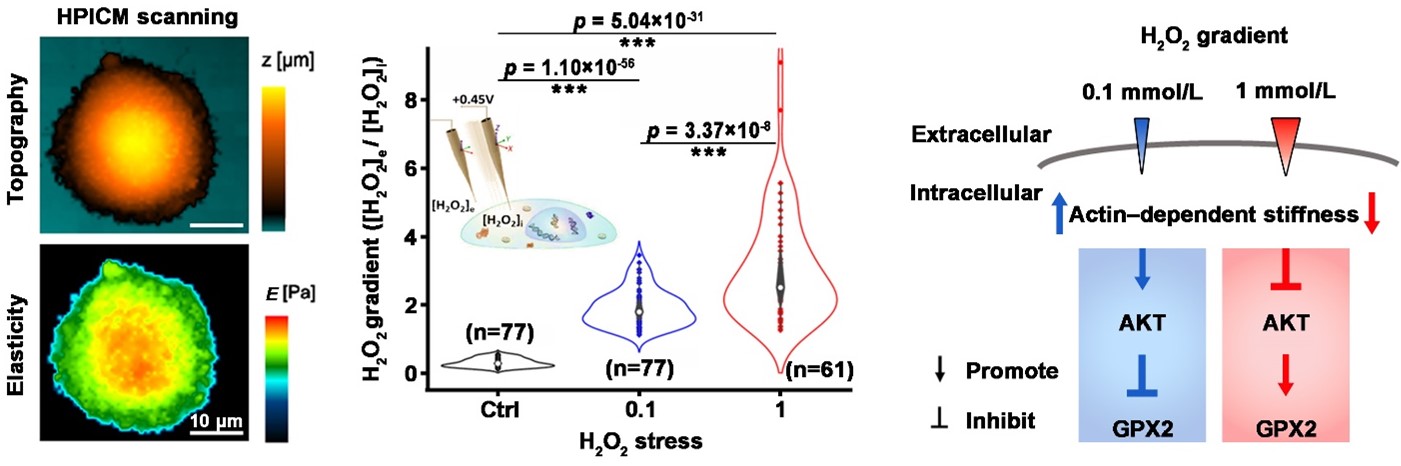 Figure. Exploring the effect of H2O2 eustress at single-cell level using hopping probe scanning ion conductance microscopy.
Figure. Exploring the effect of H2O2 eustress at single-cell level using hopping probe scanning ion conductance microscopy.
Left: The study utilizes hopping probe scanning ion conductance microscopy (HPICM) to non-invasively investigate the cellular morphology and mechanical properties of individual colorectal cancer Caco-2 cells, offering precise imaging and characterization without physical contact.
Middle: Highly sensitive Pt-functionalized carbon nanoelectrodes enable the measurement of dynamic changes in extracellular-to-intracellular H2O2 gradients of individual Caco-2 cells under H2O2 eustress conditions.
Right: The study observed that Caco-2 cells responded to H2O2 eustress at 0.1 mM by increasing cellular F-actin-dependent stiffness, but this stiffness decreased at 1 mM. Interestingly, this eustress-induced stiffness positively regulated AKT activation while negatively influencing the expression of the H2O2-scavenging enzyme GPX2. These findings reveal a novel interplay between physical properties and biochemical signaling in cancer cells’ antioxidant defense, shedding light on the exploitation of H2O2 eustress for survival at the single-cell level.
© 2024 Science China Press. Published by Elsevier B.V. and Science China Press
Glossary
※1:Hopping Probe Scanning Ion Conductance Microscopy (HPICM)
The principle of scanning ion conductance microscopy (SICM) relies on monitoring the ion current that flows through a nanopipette containing electrolyte. It can map the topography of a sample by adjusting the nanopipette height to maintain a constant ion current as it is scanned over the sample surface. The proximity to the sample surface has a measurable effect on the ion current flow and, hence, feedback-controlled nanopipette withdrawal before the nanopipette makes contact with the sample, making it useful for non-contact imaging of living biological specimens.
In this study, SICM has been used in hopping probe mode, named Hopping Probe Scanning Ion Conductance Microscopy (HPICM), where the nanopipette constantly approaches and retracts from the sample, further reducing the potential for sample damage, particularly where the sample surface is not flat. The authors demonstrated that their HPICM can detect the dynamic changes in both cellular mechanical properties and topographical morphology of individual cancer cells in real-time.
Reference
Authors: Dong Wang, Emily Woodcock, Xi Yang, Hiromi Nishikawa, Elena V Sviderskaya, Masanobu Oshima, Christopher Edwards, Yanjun Zhang, and Yuri Korchev.
Title: Exploration of individual colorectal cancer cell responses to H2O2 eustress using hopping probe scanning ion conductance microscopy.
Journal: Science Bulletin
Published on Apr. 3, 2024.
DOI: 10.1016/j.scib.2024.04.004
URL:







