As the world population hovers around 7.8 billion, staring down nearly 10 billion by 2050, one of the biggest challenges is global food security.
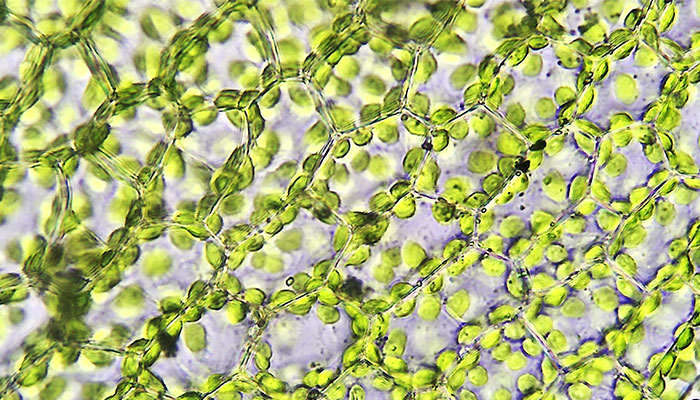
Green scene: A microscopic magnification of the structure of plant leaf cells, showing chloroplast which makes plants grow – and is the target of Macquarie University synthetic biology researchers.
The race is on to promote crops that require less and less water, and fewer agrochemicals; crops that are more nutritious, resilient to climate change, resistant to disease, and have higher yields.
The secret weapon in the quest for 21st century food security might turn out to be the humble chloroplast, the structures of a plant cell that generate its food.
We all remember the chloroplast story from high school science. The chloroplast is the driver of nature’s star process: photosynthesis, which turns sunlight into usable energy, making plants grow.
Imagine that the plant genes the pathogens are hoping to infect have left home with no forwarding address. The troublemakers are left without a target.
Writing in Nature Communications, Dr Briardo Llorente, an associate investigator in the ARC Centre of Excellence in Synthetic Biology at Macquarie University, and his colleagues propose a way to make tomorrow’s plant foods more productive, sustainable and disease-resistant by helping the chloroplast wrest back control ceded to the nucleus millions of years ago.
The chloroplast was originally a photosynthetic bacterium that lived inside a host cell. Evolution saw most of the bacterium genes either lost or transferred to the nucleus of the host cell.
As a consequence, modern chloroplasts retain a relatively small functional genome (a genome is the genetic information of an organism) of usually 100 to 250 genes. Thousands of genes that the chloroplast needs to function are now in the nuclear genome of the plant cell.
Says Llorente, “We consider reverting this evolutionary process as a new way of improving both plant disease resistance and photosynthesis.”
Target practice
To date, crop disease has been kept at bay largely with chemical control agents. And while genetic disease resistance is the most effective alternative, pathogens such as viruses, bacteria and fungi are known to adapt rapidly to reduce that resistance. Stronger measures were called for.

Sights on spuds: Because their chloroplast genomes can be reliably engineered, potatoes will be one of the first crops to be road-tested by the Macquarie University researchers.
Llorente is looking to relocate key genes from the nucleus to the chloroplast, where pathogens won’t find them. Imagine that the plant genes the pathogens are hoping to infect have left home with no forwarding address. The troublemakers are left without a target, rendering their infection strategies ineffective.
“It’s a common infection strategy among plant pathogens to disrupt the working of nuclear genes that support chloroplasts,” says Llorente. “The plant cells become manipulated and easier to infect, so that the pathogens can propagate.”
Improving photosynthesis has long been identified as the primary target with enormous potential to significantly enhance crop yields.
Llorente has identified genes that are the key targets of pathogens. “We just put the nuclear DNA sequence that corresponds to those genes into the chloroplast genome,” he explains. “Basically, it’s a new location within the cell, but it’s the same information.”
Potatoes and commercial tobacco will be the first crops to be road-tested in this way, with tomatoes and lettuces also in his team’s sights. These are all important agricultural crops whose chloroplast genomes can be reliably engineered. In the future, the number of crops that could be improved in the same way would certainly increase.
Signalling the Help Desk
In his paper for Nature Communications, titled Llorente maintains that photosynthetic processes have not been evolutionarily optimised for the conditions and needs of modern agricultural food production or to cope with changes in the global climate.
“Hence, improving photosynthesis has long been identified as the primary target with enormous potential to significantly enhance crop yields,” he writes.

A plant cell typically has one nucleus, and many chloroplasts, often about 100, all constantly identifying their needs to the nucleus. It’s like a bio help desk. However, the nucleus sends back what’s asked for to all the chloroplasts equally, instead of addressing each lodged support request individually.
In other words, says Llorente, the two-way communication that developed between the chloroplast and the nucleus has been too democratic and balanced for its own good.
“The relocation of nuclear genes to the chloroplast genome could allow each chloroplast to become more autonomous and capable of optimising its functioning and photosynthetic activities.
“Altogether, the approach could have a powerful two-fold effect – crop resistance to diseases and improved photosynthesis, leading to increased crop yields.”
is an associate investigator in the ARC Centre of Excellence in Synthetic Biology at Macquarie University






