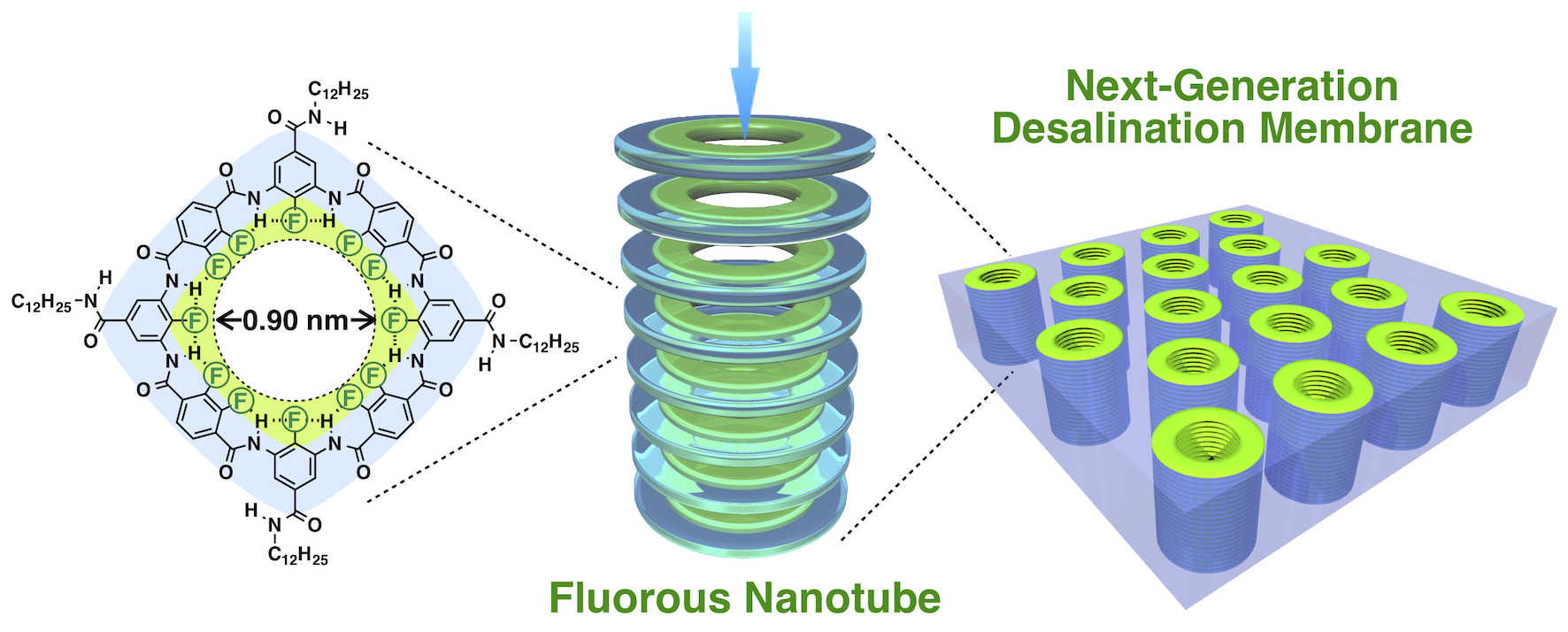
Fluorous nanotubes. Reducing the energy and thus financial cost, as well as improving the simplicity of water desalination, could help communities around the world with poor access to safe drinking water. © 2022 Itoh et al.
Water scarcity is a growing problem around the world. Desalination of seawater is an established method to produce drinkable water but comes with huge energy costs. For the first time, researchers use fluorine-based nanostructures to successfully filter salt from water. Compared to current desalination methods, these fluorous nanochannels work faster, require less pressure and less energy, and are a more effective filter.
If you’ve ever cooked with a nonstick Teflon-coated frying pan, then you’ve probably seen the way that wet ingredients slide around it easily. This happens because the key component of Teflon is fluorine, a lightweight element that is naturally water repelling, or hydrophobic. Teflon can also be used to line pipes to improve the flow of water. Such behavior caught the attention of Associate Professor Yoshimitsu Itoh from the Department of Chemistry and Biotechnology at the University of Tokyo and his team. It inspired them to explore how pipes or channels made from fluorine might operate on a very different scale, the nanoscale.
“We were curious to see how effective a fluorous nanochannel might be at selectively filtering different compounds, in particular, water and salt. And, after running some complex computer simulations, we decided it was worth the time and effort to create a working sample,” said Itoh. “There are two main ways to desalinate water currently: thermally, using heat to evaporate seawater so it condenses as pure water, or by reverse osmosis, which uses pressure to force water through a membrane that blocks salt. Both methods require a lot of energy, but our tests suggest fluorous nanochannels require little energy, and have other benefits too.”
The team created test filtration membranes by chemically synthesizing nanoscopic fluorine rings, which were stacked and embedded in an otherwise impermeable lipid layer, similar to the organic molecules that make up cell walls. They created several test samples with nanorings between about 1 and 2 nanometers. For reference, a human hair is almost 100,000 nanometers wide. To test the effectiveness of their membranes, Itoh and the team measured the presence of chlorine ions, one of the major components of salt – the other being sodium – on either side of the test membrane.
“It was very exciting to see the results firsthand. The smaller of our test channels perfectly rejected incoming salt molecules, and the larger channels too were still an improvement over other desalination techniques and even cutting-edge carbon nanotube filters,” said Itoh. “The real surprise to me was how fast the process occurred. Our sample worked around several thousand times faster than typical industrial devices, and around 2,400 times faster than experimental carbon nanotube-based desalination devices.”
As fluorine is electrically negative, it repels negative ions such as the chlorine found in salt. But an added bonus of this negativity is that it also breaks down what are known as water clusters, essentially loosely bound groups of water molecules, so that they pass through the channels quicker. The team’s fluorine-based water desalination membranes are more effective, faster, require less energy to operate and are made to be very simple to use as well, so what’s the catch?
“At present, the way we synthesize our materials is relatively energy-intensive itself; however, this is something we hope to improve upon in upcoming research. And, given the longevity of the membranes and their low operational costs, the overall energy costs will be much lower than with current methods,” said Itoh. “Other steps we wish to take are of course scaling this up. Our test samples were single nanochannels, but with the help of other specialists, we hope to create a membrane around 1 meter across in several years. In parallel with these manufacturing concerns, we’re also exploring whether similar membranes could be used to reduce carbon dioxide or other undesirable waste products released by industry.”




