The well-known representation of chemical elements is just one example of how objects can be arranged and classified
The periodic table of elements that most chemistry books depict is only one special case. This tabular overview of the chemical elements, which goes back to Dmitri Mendeleev and Lothar Meyer and the approaches of other chemists to organize the elements, involve different forms of representation of a hidden structure of the chemical elements. This is the conclusion reached by researchers at the Max Planck Institute for Mathematics in the Sciences in Leipzig and the University of Leipzig in a recent paper. The mathematical approach of the Leipzig scientists is very general and can provide many different periodic systems depending on the principle of order and classification – not only for chemistry, but also for many other fields of knowledge.
It is an icon of natural science and hangs in most chemistry classrooms: the periodic table of elements, which is celebrating its 150th birthday this year. The tabular overview is closely linked to Dmitri Mendeleev and Lothar Meyer – two researchers who, in the 1860s, created an arrangement of elements based on their atomic masses and similarities. Today they are sorted by atomic number (which indicates the number of protons in the atomic nucleus) from the light hydrogen (one proton) to the exotic oganesson (118 protons). The elements are also classified into groups: Atoms in the same column usually have the same number of electrons in their outer shell.
Periodic table in different variants

At first glance, the periodic table seems to have brought an unambiguous and final order to the currently known 118 elements. But appearances can be deceptive because many things still remain controversial: Scientists do not agree on exactly which elements belong in the third group below scandium and yttrium. For example, the correct position of lanthanum and actinium is debated. If one takes a closer look, one will discover slightly different variants of the periodic table in classrooms, lecture halls, and textbooks.
Guillermo Restrepo and Wilmer Leal from the Max Planck Institute for Mathematics in the Sciences and the University of Leipzig are not surprised. For them, there is no unambiguously correct arrangement of the elements; depending on the criterion applied for classification, a different periodic table results. The atoms can be subdivided according to the electron configuration (i.e. the number and arrangement of their electrons), their chemical behaviour, their solubility, or their occurrence in geological deposits. It is now widely accepted that the chemical elements should be arranged according to their atomic number and divided into groups according to their electron configuration. But even for this periodic table, there are numerous different forms of representation. For example: as a spiral with various bulges, pyramid-shaped, or as a three-dimensional flower.
A common structure behind the periodic tables
Guillermo Restrepo and Wilmer Leal have now systematically investigated the ambiguity of the periodic table. This has led to findings that are also of considerable importance beyond chemistry. Accordingly, all forms of representation of the chemical elements are based on a common structure, which mathematicians refer to as an ordered hypergraph. The venerable periodic table of Mendeleev and Meyer thus offers only a representation of the general structure, which Guillermo Restrepo and Wilmer Leal now postulate. New arrangements can also be derived from this at any time. Guillermo Restrepo therefore compares the order of the chemical elements with a sculpture on which light falls from different directions. “The various shadows that the figure casts are the periodic tables. That’s why there are so many ways to create these tables. In a way, the period tables are projections. Projections of the internal structure of the periodic table”.
The scientists from Leipzig are now trying to determine the hidden mathematical structure on which the known periodic tables of chemistry are based. For the time being, they have defined three conditions that must be met in order to establish a periodic table. First, one needs objects that are to be ordered. For Mendeleev, Meyer and the creators of the other known periodic tables of chemistry, these are the chemical elements. These objects must be arranged according to some properties such as the atomic mass or the atomic number (i.e. the number of protons). Finally, one criterion is required to group the objects in classes. Mendeleev and Meyer used the chemical similarity for this.
Periodic table of chemical bonds
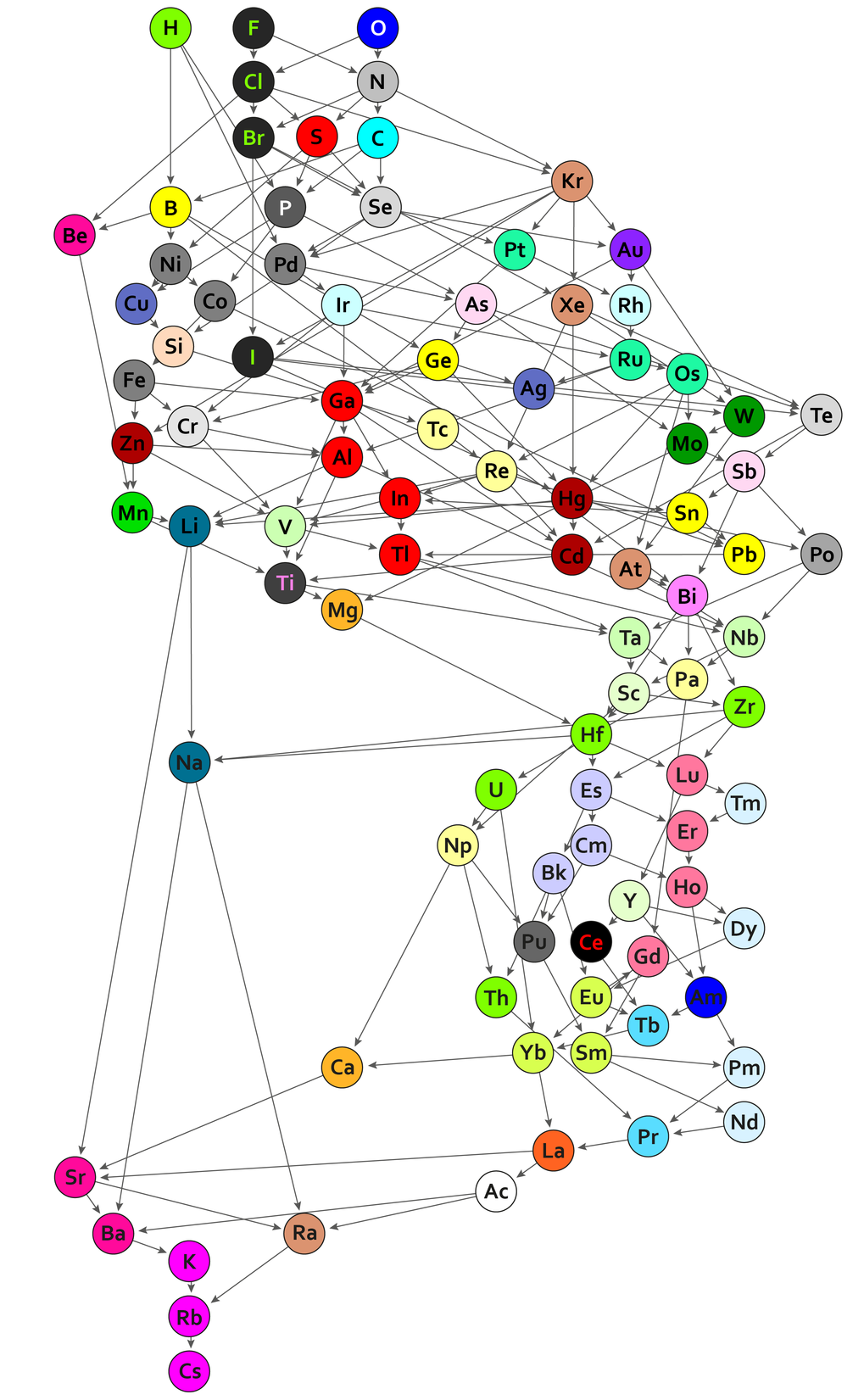
“If these three conditions are met, periodic tables can also be created for other chemical objects and even for objects outside chemistry”, says Guillermo Restrepo. He and Wilmer Leal show this by looking at the chemical bonds between atoms of 94 elements and different conjugates. The polarizability of 94 single-covalent bonds, where bonds are arranged according to the electronegativity and atomic radius of one of the bonded atoms. For example, fluorine, chlorine, or oxygen are highly electronegative and assume relatively small atomic radii in compounds. The bonds are then classified based on how much they resemble each other.
“We have investigated almost 5,000 substances consisting of two elements in different proportions”, explains Guillermo Restrepo. “We then looked for similarities within this data. For example, sodium and lithium are similar because they combine with the same elements in the same proportions (e.g. with oxygen or chlorine, bromine, and iodine). We thus found patterns we can use to classify the elements”.
A periodic table as a network instead of a matrix
In the 44 classes of chemical elements, there are some similarities with the main groups of Mendeleev’s and Meyer’s periodic table. For example, the alkali metals sodium and lithium are found in one group because they form the same simple salts with halogens such as chlorine or fluorine. Like the elements themselves, the bonds of the four halogens (fluorine, chlorine bromine, and iodine) are also found in the same group. However, there are also classifications that differ significantly from those in the conventional periodic table. For example, carbon and silicon are no longer in the same class because they form very different compounds.
The representation of the periodic table of chemical bonds also has nothing to do with the familiar matrix-like arrangement of the classical periodic tables of the elements. Instead, the 94 covalent bonds are represented in a network of differently coloured circles. Each circle represents a chemical bond, and the colour symbolizes belonging to one of the 44 groups. Because now two criteria are used for the sorting, there is no longer any clear order of the atoms (like in the tables of Mendeleev and Meyer) – mathematicians speak of a partial order. The circles are therefore connected to other circles by one or more arrows, thereby creating an ordered hypergraph.
Periodic tables in other scientific fields
The chemical elements and their compounds can also be represented in completely different periodic tables – depending on the underlying order and classification principle. What’s more: The objects of numerous other scientific fields and their applications can also be arranged in periodic tables. For example, ordered hypergraphs are used in information systems and web mining. Possible periodic systems also emerge when countries are considered; these can be classified according to social or economic indicators as well as geographical proximity or cultural similarity. Other examples can be found in engineering, environmental sciences, sociology, and many other disciplines. The scientists not only study periodic systems because of their importance for chemistry, but, above all, because of their applications in many other disciplines.







