A messenger molecule keeps dendritic cells together on their patrol through the tissue
Dendritic cells can be used to predict the course of cancer: the more of them there are in a tumor, the better the prospects for the patient. The cells of the immune system circulate primarily in the blood and migrate into the body’s tissues after inflammation. Some types of immune cells, however, reside permanently in the tissues, where they assemble into three-dimensional networks. A team led by Wolfgang Kastenmüller, Georg Gasteiger and Dominic Grün from the Max Planck Research Group for Systems Immunology and the Julius Maximilians University of Würzburg has now studied this particular type of immune cell in more detail.
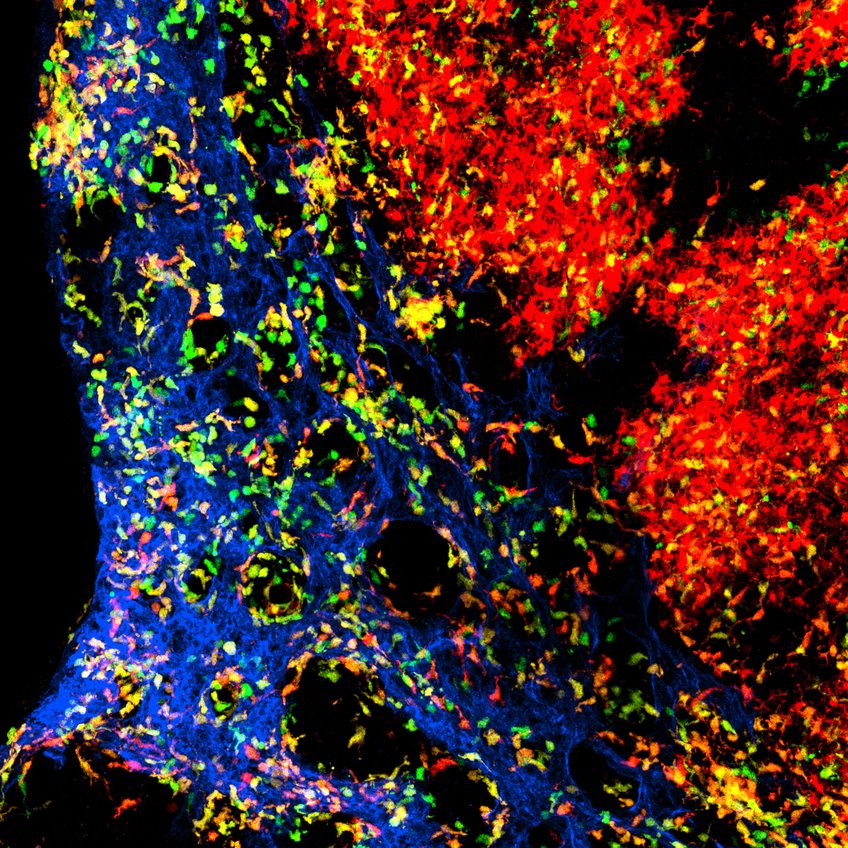
The figure shows the complex organization of dendritic cells in the lymph node. Blood vessels are shown in blue. The cells in green are young dendritic cells whereas the dendritic cells in red are a few days older and have already migrated. The dendritic cells in orange are intermediate in age.
© Milas Ugur/ Universität Würzburg
Dendritic cells are essential for controlling immune responses as they reside at the frontline of the immune system. They recognize foreign structures, capture them, and process them into a kind of “wanted poster.” They then present this poster to other immune cells, triggering a specific immune reaction, such as against pathogens or cancer cells.
What makes dendritic cells special is that they have a lifespan of only about a week and continuously migrate through the body’s tissues during this time. “In this regard, it was clear that the classical niche concept, as known from macrophages for example, does not apply here,” says Wolfgang Kastenmüller from the Max Planck Research Group for Systems Immunology. The research team discovered a completely novel concept in animal models, where three-dimensional cell networks can organize: dendritic cells align themselves with blood vessels and migrate in a single-file fashion along their outer walls, similar to children walking in a line. Therefore, the blood vessels determine the three-dimensional arrangement of the cells. “We wanted to understand how this process is regulated and how the cells manage to fill gaps in their collective,” explains Milas Ugur, postdoc in Wolfgang Kastenmüller’s research group. Closing such gaps is important because otherwise, the immune defense would no longer function optimally.
As reported by the team, it is a locally acting messenger molecule called FLT3 ligand that keeps the dendritic cells together during their migration. This messenger molecule, and presumably others, are continuously produced on-site and consumed by the dendritic cells. If there are gaps in the collective, the isolated dendritic cells have access to more messenger molecules. This surplus accelerates their development and movement, helping them catch up with the group. Once the cells have caught up, due to competition from their neighbors, they have slightly fewer messenger molecules available. Consequently, they slow down their rate of development.
Avenues for immunotherapy
These findings are particularly relevant in the context of cancer therapy. Dendritic cells hold high prognostic value for tumor diseases: The more dendritic cells present in a tumor, the better the prospects for the patients. This is especially true after immunotherapy. “With a comprehensive understanding of dendritic cells through fundamental knowledge, we can better comprehend how to restore the networks of these cells within tumors. This knowledge will enable the development of personalized therapies in the future,” explains Kastenmüller. The existing data from the research group is based on the analysis of lymph nodes from animal models. The team’s next goal is to test whether the same principles of network organization of dendritic cells apply to all tissues, including humans.








