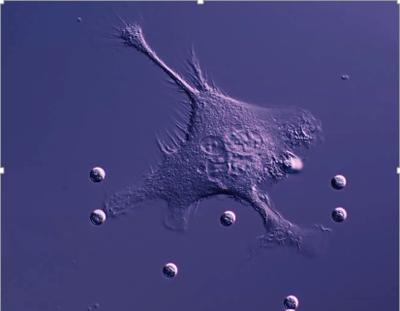
Scientists at the University’s Centre for Cancer Immunology have begun a new collaborative study to understand how therapeutic antibodies stimulate the body’s immune cells to kill other cells that contribute to a wide range of diseases, including cancer.
Working with researchers at South Dakota State University, the team will examine how specialized immune cells called macrophages, recognize and destroy target cells and why they sometimes do not.
The results will help increase the effectiveness of antibody therapeutics designed to fight cancer and target autoimmune disorders, such as multiple sclerosis and rheumatoid arthritis.
Macrophages are able to recognise target cells that are ‘tagged’ for destruction by antibodies bound to the target cell surface however, sometimes the macrophages either do not respond or actively clean the antibody off the target cell and then let it go—they do not kill it. This deactivation makes the antibody therapeutic ineffective and allows the target cells to escape.
The team at South Dakota State University will use the CRISPR gene-editing tool to identify which genes promote or inhibit the macrophages’ ability to use antibodies to destroy target cells. They will also use powerful computational bioinformatics approaches to analyse which genes are expressed in the different circumstances.
The Southampton research group, composed of Professor Mark Cragg alongside Professor Stephen Beers and Dr Stephen Thirdborough, will then study the genes identified through the CRISPR screenings in mice experiments to verify their importance. In addition, they will analyse data from patients who received antibody therapeutics to treat cancer and autoimmune disorders to confirm their results.
Professor Cragg said: “Antibody therapies can be very powerful – however, sometimes the immune cells stop killing the disease-causing cells. We want to understand the pathways and immune signals that control macrophage activity to allow us to make them more potent killers of cancer cells.”
The five-year $1.78 million study is funded by the ³Ô¹ÏÍøÕ¾ Institutes of Health in the USA and will involve postdoctoral researchers and doctoral students. It is one of six projects that were funded as part of a focused effort aimed at improving the effectiveness of therapeutic antibodies— and one of the few that involves an international collaboration.
Professor Adam Hoppe, of South Dakota State University, said: “We want to understand what is deactivating the macrophages and we want to know why those antibodies can perform so well in some patients, but fail in others.”
Speaking about working with the he added: “They are the world’s experts on therapeutic antibodies that can target autoimmune or malignant B and T cells,” noting that they recently discovered how immune activation of macrophages is one the key components for effective antibody therapeutics.