Cyclic peptides often exhibit low membrane permeability which can be significantly improved via amide-to-ester substitutions—as demonstrated by researchers from Tokyo Institute of Technology (Tokyo Tech). The utilization of substitutions shown in this study can be used to develop cyclic peptides with high membrane permeability and oral bioavailability for clinical and therapeutic applications.
Interest in cyclic peptides, a class of organic molecules, has reached a new high recently. Their ability as inhibitors has made them potential candidates for clinical and therapeutic applications. Unlike their linear counterparts, peptides with macrocyclization have better resistance to proteolysis—the disintegration of peptides into amino acids in the presence of enzymes—which makes them stable in the bloodstream—desired property for any material being used for therapeutic purposes.
However, the membrane permeability—the ability of a molecule to pass through cell membranes in our body—of cyclic peptides is quite low in general. This downside not only makes formulation of orally bioavailable peptides difficult, but also severely affects their ability to target intracellular protein interactions. On the other hand, some natural cyclic peptides show great membrane permeability and oral bioavailability. These naturally occurring membrane-permeable cyclic peptides often have N-methylamide bonds, ester bonds, or both on their backbone, instead of amide bonds those are the most common for amino acids in proteins and peptides. While studies have explored the effects of N-methylation on cyclic peptides, the impact of amide-to-ester substitution remains unexplored.
That was until a multinational and interdisciplinary group of researchers from Tokyo Tech, The University of Tokyo, and University of California, Santa Cruz, came together to discover the unknown. In their recent breakthrough published in , the team reported a direct evaluation of amide-to-ester substitutions and their effect on the membrane permeability of cyclic peptides. Prof. Yutaka Akiyama from the Tokyo Tech, a corresponding author of this paper explains, “We know that naturally occurring depsipeptides, which are peptides where one or more of the amide bonds are replaced with an ester bond, show high membrane permeability. This is why our team decided to see if amide-to-ester substitutions can improve membrane permeability. In the team, Tokyo Tech scientists employed a newly developed method on enhanced sampling molecular dynamics (MD) simulations to unravel the mechanism behind this.”
The team first synthesized a series of model dipeptides and their derivatives containing amide-to-ester substitutions. They then compared the membrane permeability of these peptides using wet lab techniques and found that the substituted dipeptides had significantly higher membrane permeability (Figure 1, Left).
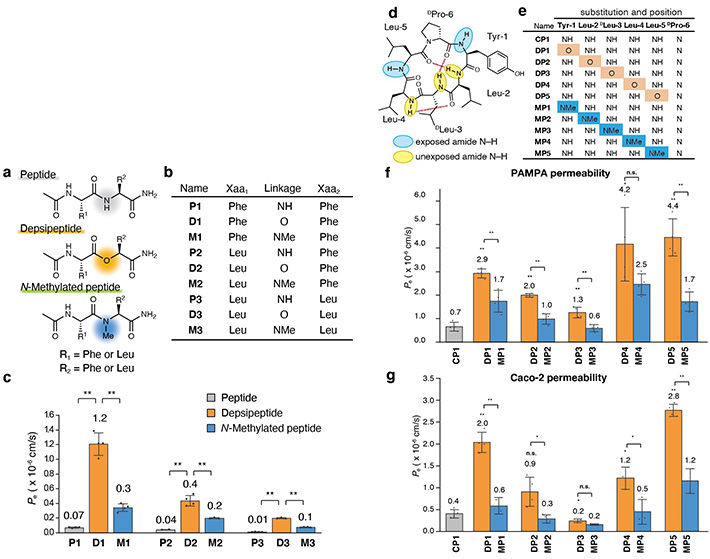
Figure 1. Analyzing the effect of amide-to-ester substitution on the permeability of cyclic peptides.
(Left) (a) Chemical structures of model dipeptides. (b) List of dipeptide molecules synthesized. (c) Parallel artificial membrane permeability assay (PAMPA) of the dipeptides.
(Right) (d) Chemical structures of model cyclic hexapeptides. (e) List of hexapeptide molecules synthesized. (f)&
(g) PAMPA and Caco-2 assay of the synthesized cyclic hexapeptides.
To better understand the scope of these substitutions, the team tried amide-to-ester replacements on larger cyclic hexapeptides. The findings indicated that amide-to-ester substitution enhanced the membrane permeability of cyclic hexapeptides much more than the conventional N-methylation process (Figure 1, Right).
The team also carried out enhanced sampling MD simulations on three cyclic hexapeptides (CP1, DP1, and MP1) using the at Tokyo Tech. The large-scale simulations revealed that the substituted peptides dynamically transition between their open and closed conformations in aqueous solution and at the membrane interface (Figure 2). They also revealed that the amine-to-ester substitution led to a corresponding reduction in the free energy barrier for the substituted peptides, thereby leading to their enhanced membrane permeability.
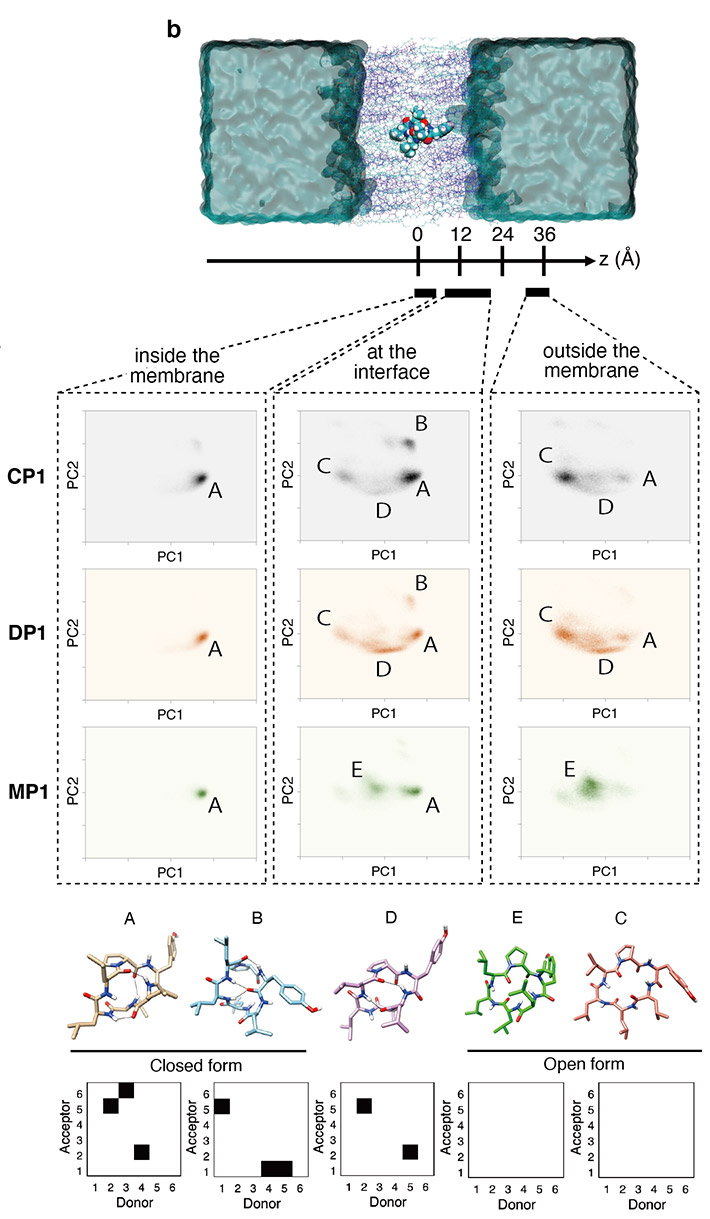
Figure 2. Enhanced sampling MD simulations of the membrane permeation process of cyclic peptides.
(Top) Conformational ensembles of three cyclic peptides (CP1, DP1, and MP1) inside, at the interface, and outside the lipid membrane projected onto the first and second principal components (PC1 and PC2).
(Bottom) Five representative peptide conformations (including closed and open forms) and corresponding backbone hydrogen bonding patterns.
The insights provided by this study could help expand the utility of cyclic peptides by providing a simple solution to the low permeability problem. “We envision that this amide-to-ester substitution strategy will be utilized for the development of peptides with better oral bioavailability, ability to target intracellular biomolecules, or both,” concludes Prof. Akiyama.
Reference
Authors : | Yuki Hosono1, Satoshi Uchida 1, Moe Shinkai1, Chad E. Townsend2, Colin N. Kelly2, Matthew R. Naylor 2, Hsiau-Wei Lee2, Kayoko Kanamitsu3, Mayumi Ishii3, Ryosuke Ueki 1, Takumi Ueda 3, Koh Takeuchi 3, Masatake Sugita4,5, Yutaka Akiyama 4,5, Scott R. Lokey2, Jumpei Morimoto 1, Shinsuke Sando 1,6 |
Title : | Amide-to-ester substitution as a stable alternative to N-methylation for increasing membrane permeability in cyclic peptides |
Journal : | Nature Communications |
DOI : | |
Affiliations : | 1 Department of Chemistry and Biotechnology, Graduate School of Engineering, The University of Tokyo, Japan 2 Department of Chemistry and Biochemistry, University of California, Santa Cruz, USA 3 Graduate School of Pharmaceutical Sciences, The University of Tokyo, Japan 4 Department of Computer Science, School of Computing, Tokyo Institute of Technology, Japan 5 Middle-Molecule IT-based Drug Discovery Laboratory (MIDL), Tokyo Institute of Technology, Japan 6 Department of Bioengineering, Graduate School of Engineering, The University of Tokyo, Japan |








