Every year about 1.7 million heart pacemakers are fitted globally, with more than 30,000 in Australia helping to dramatically improve recipients’ health expectations. Now a South Australian cardiovascular monitoring device company is helping to take the guesswork out of pacemaker surgery and even extend the life of the pacemaker’s efficiency by selling its OnePoint Junction Box in 26 countries as sales head for $1 million.
After turnover of 500 units of the first electrogram device to hospitals, heart specialists and multinationals such as Boston Scientific, Biotronik and Medtronic, Tonsley-based C.tech (or Cardiovascular Technology Pty Ltd) is focusing on expanding its product range with a $600,000 capital raising and staff expansion.
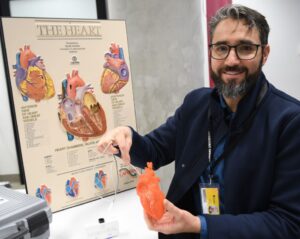
Founder and managing director Darius Chapman, with chief medical adviser Professor Anand Ganesan – a cardiac clinician and electrophysiologist based at Flinders, saying the new TorqView device will put the four-year old company on a strong growth trajectory.
“These devices are impressing pacemaker implant physicians and medical device companies by bringing a new level of accuracy to routine and sometimes critical pacemaker surgeries,” says Flinders University Cardiology Research Fellow Mr Chapman, a Flinders graduate in electrical and electronics engineering.
“Our federal and state accelerator business grants have helped launch the technology, which has brought cutting-edge technology to redefine cardiac conduction pacing to revolutionise this sector of the $10 billion medical device world market.”
is designed to simplify cable connections and support secure connections in conduction system pacing, helping surgeons to more accurately position the pacemaker to the heart.
The next-gen TorqView device is seeking ISO 13485 Quality Management System certification for crucial monitoring of pacemaker signals during surgery to meet another unmet need in cardiac care when it’s launched in 2024, with support from the Flinders University Medical Device Research Institute at Tonsley.
C.tech has now relocated to ) at the Tonsley Innovation District for its next stage of ramping up production and growth. New staff members include Flinders cardiology researcher Campbell Strong, head of manufacturing Ryan Jolly and quality controller Aby Jose who will collaborate with other technology startups in the district to strengthen the medical device hub at Tonsley.

“Being part of this vibrant innovation district offers us an ideal opportunity to collaborate with a diverse community of innovators, academics, and business leaders,” Mr Chapman says.
“This dynamic environment will inspire us to push the boundaries of what’s possible in cardiac conduction pacing, providing us with the resources and opportunities to accelerate our growth.”
The NVI entrepreneur centre at Tonsley also has world-class 3D printing and other design and manufacture infrastructure, business networking and advisory services as well as contacts with other Flinders clinicans and Flinders University biomedical and mechanical engineering and IT academics.
Acknowledgements: C.tech received funding from the Australian Government Accelerating Commercialisation grant program and has worked with the Medical Device Partnering Program and New Venture Institute based at Flinders University, Tonsley Innovation District .








