Key Points
Experiments on Earth have confirmed that clusters formed by lighter charged particles in the microgravity environment of space are 50% larger than clusters formed on earth
Controlling the self-assembly of colloidal particles enables the construct of novel materials for multiple applications including photonics and drug design
The neutron scattering instruments Quokka and Kookaburra provided unparalleled information on the structure of clusters
A study conducted by group of scientists from Nagoya City University (NCU), Japan Space Forum (JSF), Advance Engineering Services (AES), Japan Aerospace Exploration Agency (JAXA) and ANSTO has revealed a clustering of charged particles in the microgravity environment of International Space Station (ISS), with implications for the development of photonic materials, better drugs, and a range of new and innovative materials that depend on the mixing of two or more charged particles.
The which was published in Nature – Microgravity, conducted on the ISS determined how sub-micron sized charged colloidal particles interact in the presence and absence of earth’s gravity.
“Many chemical and physical phenomena rely heavily on an understanding of how two particles interact with each other, especially charged particles,” said ANSTO senior scientist and co-author, Dr Jitendra Mata.
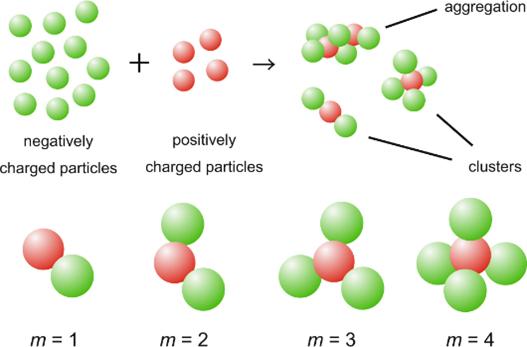
“The best example is when colloidal particles form tetrahedral clusters, commonly known as diamond lattices, which are essential in producing photonic materials. Controlling the self-assembly of colloidal particles enable us to construct a novel material that can be used in photonic, optoelectronics, sensing and clinical diagnostics.”
It is well known that even the slightest gravitational sedimentation and convection on Earth affects particle interactions and their arrangement in a colloid. This hinders important knowledge about the effect of charge.
This knowledge can also help to design better drug formulations, which will have higher self-life and better efficacy.
In this study researchers selected positively and negatively charged lighter particles and heavy particles. Polystyrene particles are only as heavy as the aqueous medium that contains them, and titania particles are roughly 3 times heavier than the medium.
Samples were immobilised in a gel after their interaction so they could be brought back to Earth for various experiments.
The research revealed that clusters formed by lighter particles in space are 50% larger than clusters formed on earth. This is ground-breaking finding as it was not expected for lighter particles.
For heavy particles, such as titania, an electrostatic interaction and cluster formation was also confirmed which it is not possible at all on Earth.
This study also needed an engineering marvel, in terms of designing the experimental setup for the mixing of samples in space and immobilising these samples after mixing.
After the project was selected by JAXA, the team worked closely with multiple organisations to make a custom-built setup which can allow for the mixing and immobilising of clusters in gel using LED-UV light.
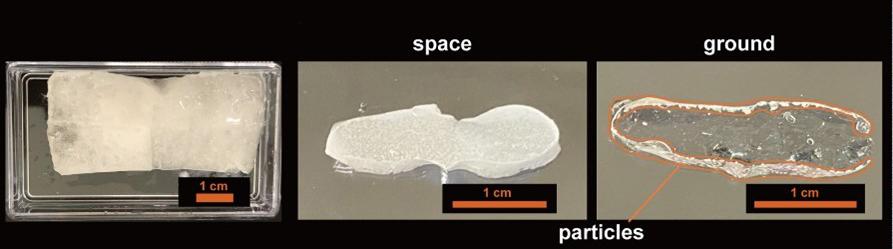
Two sets of samples were prepared in Japan; one was sent to ISS using a Falcon rocket (Space-X) and Dragon SpX-19 transporter and other was used in a ground experiment. The ISS crew used the prescribed procedure to mix the samples before curing them with LED-UV light. After spending more than a year in space, samples were return to Earth and were sent to different institutes for analysis.
A set of samples came to ANSTO, which is home to two state-of-the art reactor based instruments – Small Angle Neutron Scattering (SANS) -and – Ultra Small Angle Neutron Scattering (USANS).
“Quokka and Kookaburra are unique instruments which provided unparalleled information on the structure of clusters, which is very hard to study by other techniques. With contrast variation SANS and USANS, it was possible to gain information on the individual components in the clustering process,” said Dr Mata.
Combined data from these two instruments provided important knowledge of structural morphology and the charge-charge interaction of colloidal particles from ~1nm to 10 µm, without compromising the crystal environment of the samples. The study also features many other techniques including mathematical modelling and simulations.







