Researchers at Tohoku University have discovered a new approach for treating lymph node metastasis. Anticancer drugs are administered directly into the LNs under ultrasound guidance (Lymphatic Drug Delivery System or LDDS) to target sentinel lymph nodes (LNs) and generate antitumor effects locally, preventing distant metastasis. This approach not only improves the anticancer effect but also reduces the nasty side effects commonly associated with systemic chemotherapy.
Details of the researchers’ breakthrough were published in the journal Biomedicine & Pharmacotherapy on January 2, 2024. Professor Tetsuya Kodama from Tohoku University’s Graduate School of Engineering led the research team.
Recent research has highlighted sentinel LNs as the gateway for metastasis. They are the initial lymph nodes that receive drainage from a primary tumor and indicate that metastasis has begun or could potentially occur. Thus, there is a need for improved control of LN metastasis without surgical or radiation interventions.
Still, the occurrence of side effects can differ from individual to individual and from one treatment to another. In fact, two individuals undergoing the same treatment may encounter markedly different experiences. Most commonly, some individuals experience uncomfortable side effects that gradually diminish over time.
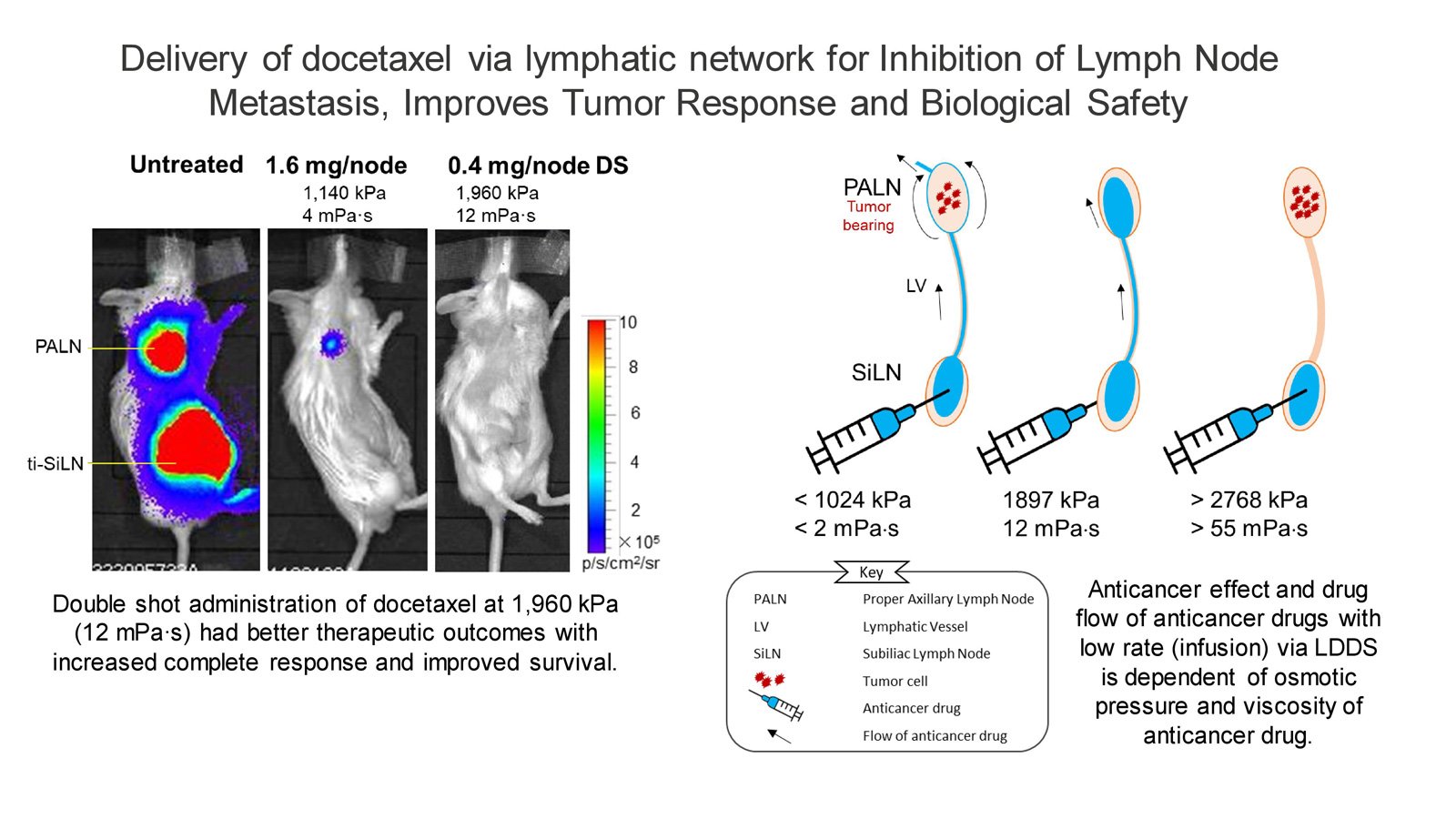
In a prior investigation, some of the current researchers utilized LDDS incorporating heightened osmotic pressure and viscosity of docetaxel, yielding promising outcomes in the treatment of early-stage LN metastasis during preclinical research. Based on this, they hypothesized that administering docetaxel – a widely used clinical anticancer drug – through LDDS at an osmotic pressure and viscosity of 1,960 kPa and 12 mPa∙s would result in superior antitumor effects, prolonged survival, and minimal adverse reactions.
To test this hypothesis, Kodama and his team administered the treatment to a preclinical model of a metastatic lymph node mouse model. Their findings confirmed superior therapeutic outcomes, increased complete response, improved survival, and reduced adverse complications. The double-shot administration enhanced T helper cell differentiation in the spleen and sentinel LNs, emphasizing its potential in cancer management. Histopathological assessments confirmed the efficacy, highlighting the potential of LDDS in cancer management.
The research team emphasizes the safer nature of the new method. “LDDS is a groundbreaking method to improve treatment while decreasing side effects,” says Ariunbuyan Sukhbaatar, assistant professor at the Graduate School of Dentistry and lead author of the paper. “This approach provides a safer alternative to LN removal and promises to improve cancer survival rates.”
Meanwhile, Kodama says, “LDDS offers a novel, safe method for delivering anticancer drugs to susceptible LNs, presenting a promising approach for improving cancer treatment effectiveness while minimizing side effects.”
Looking ahead, the research team aims to further investigate the LDDS with the goal of enhancing therapeutic responses against lymph node metastasis. This will be achieved through clinical trials conducted in collaboration with medical professionals, aiming to validate the effectiveness of these approaches in cancer patients.
- Publication Details:
Title: Docetaxel administered through a novel lymphatic drug delivery system (LDDS) improved treatment outcomes for lymph node metastasis
Authors: Ariunbuyan Sukhbaatar, Shiro Mori, Tsuyoshi Sugiura, Tetsuya Kodama
Journal: Biomedicine & Pharmacotherapy
DOI:








