Researchers develop an artificial chloroplast
For hundreds of millions of years plants have had the ability to harness carbon dioxide from the air using solar energy. The Max Planck research network MaxSynBio is on the trail of building artificial cells as sustainable green bioreactors. A Max Planck research team led by Tobias Erb from the Institute for Terrestrial Microbiology in Marburg has now succeeded in developing a platform for the automated construction of cell-sized photosynthesis modules. The artificial chloroplasts are capable of binding and converting the greenhouse gas carbon dioxide using light energy.
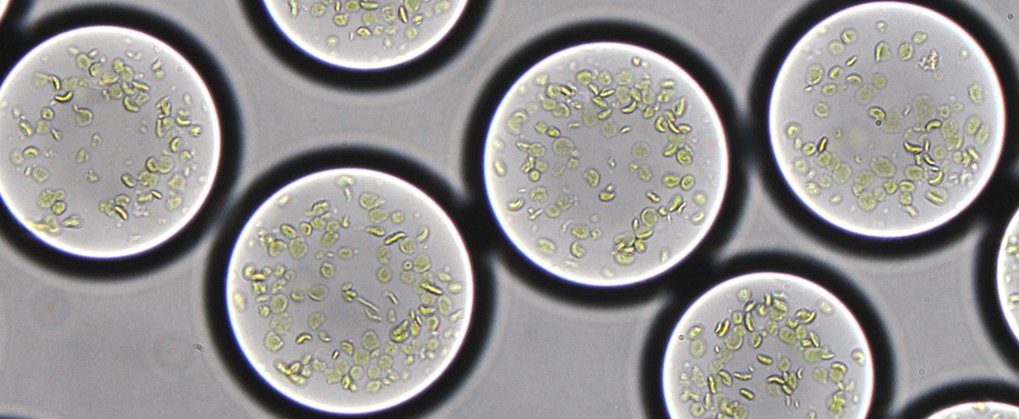
Over billions of years, microorganisms and plants evolved the remarkable process we know as photosynthesis. Photosynthesis converts sun energy into chemical energy, thus providing all life on Earth with food and oxygen. The cellular compartments housing the molecular machines, the chloroplasts, are probably the most important natural engines on earth. Many scientists consider artificially rebuilding and controlling the photosynthetic process the “Apollo project of our time”. It would mean the ability to produce clean energy – clean fuel, clean carbon compounds such as antibiotics, and other products simply from light and carbon dioxide.
But how to build a living, photosynthetic cell from scratch? Key to mimicking the processes of a living cell is to get its components to work together at the right time and place. At the Max Planck Society, this ambitious goal is pursued in an interdisciplinary multi-lab initiative, the MaxSynBio network. Now the Marburg research team led by director Tobias Erb has succeeded successfully created a platform for the automated construction of cell-sized photosynthetically active compartments, “artificial chloroplasts”, that are able to capture and convert the greenhouse gas carbon dioxide with light.
Microfluidics meets Synthetic Biology
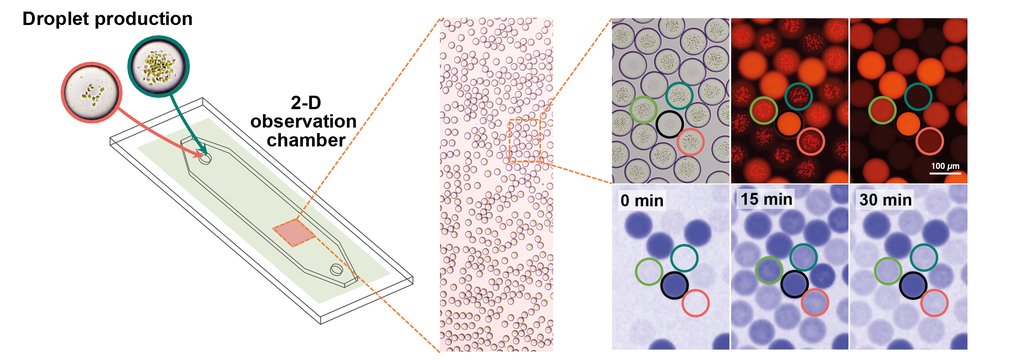
Micro-droplet production and real-time observation on a microfluidic platform. Micro-droplets are collected in a chamber where their activity can be microscopically monitored in real time, including quantifying the enzymatic activity by measuring NADPH fluorescence. Using the bright field the droplets are located and the photosynthetically active membranes can be seen. These membranes are fluorescent when excited. The droplet populations are distinguished using a coding dye, which is observable when the droplets are excited by a specific wavelength (550 nm). The NADPH production of the droplets is observed using NADPH fluorescence (using, 365 nm).
The Max Planck researchers made use of two recent technological developments: first synthetic biology for the design and construction of novel biological systems, such as reaction networks for the capture and conversion of carbon dioxide, and second microfluidics, for the assembly of soft materials, such as cell-sized droplets. “We first needed an energy module that would allow us to power chemical reactions in a sustainable fashion. In photosynthesis, chloroplast membranes provide the energy for carbon dioxide fixation, and we planned to exploit this ability “, Tobias Erb explains.
The photosynthesis apparatus isolated from the spinach plant proved to be robust enough that it could be used to drive single reactions and more complex reaction networks with light. For the dark reaction, the researchers used their own artificial metabolic module, the CETCH cycle. It consists of 18 biocatalysts that convert carbon dioxide more efficiently than the carbon metabolism naturally occurring in plants. After several optimization rounds, the team succeeded in light-controlled fixation of the greenhouse gas carbon dioxide in vitro.
The second challenge was the assembly of the system within a defined compartment on a micro scale. With a view to future applications, it should also be easy to automate production. In cooperation with Jean-Christophe Baret’s laboratory at the Centre de Recherché Paul Pascal in France, researchers developed a platform for encapsulating the semi-synthetic membranes in cell-like droplets.
More efficient that Nature’s photosynthesis
The resulting microfluidic platform is capable of producing thousands of standardized droplets that can be individually equipped according to the desired metabolic capabilities. “We can produce thousands of identically equipped droplets or we can give specific properties to individual droplets,” said Tarryn Miller, lead author of the study. “These can be controlled in time and space by light.”
In contrast to traditional genetic engineering on living organisms, the bottom-up approach offers decisive advantages: It focuses on minimal design, and it is not necessarily bound to the limits of natural biology. “The platform allows us to realize novel solutions that nature has not explored during evolution,” explains Tobias Erb. In his opinion, the results hold great potential for the future. In their study, the authors were able to show that equipping the artificial chloroplast with the novel enzymes and reactions resulted in a binding rate for carbon dioxide that is 100 times faster than previous synthetic-biological approaches. “In the long term, life like systems could be applied to practically all technological areas, including material science, biotechnology and medicine – we are only at the beginning of this exciting development.” Furthermore, the results are another step towards overcoming one of the greatest challenges of the future: the ever-increasing concentrations of atmospheric carbon dioxide.






