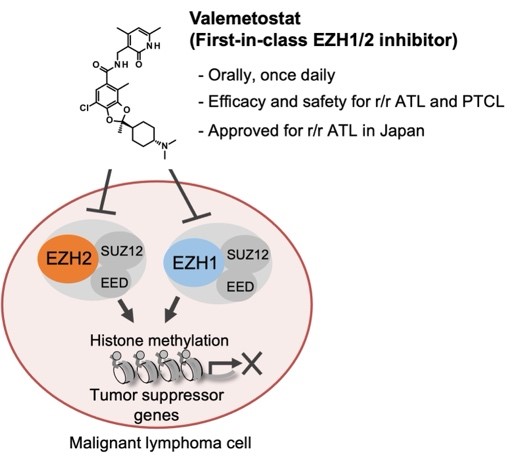
Mechanism of methylated-histone inhibitor valemetostat
Researchers revealed the mechanism of the cancer drug valemetostat and established its efficacy in treating adult T-cell leukemia/lymphoma (ATL). ©2024 Makoto Yamagishi, The University of Tokyo
Researchers have revealed the mechanism of a drug shown to be effective in treating certain types of cancer, which targets a protein modification silencing the expression of multiple tumor suppressor genes. They also demonstrated in clinical trials the efficacy of the drug in reducing tumor growth in blood cancer. The findings could lead to longer-term treatments for the disease and therapies for other types of cancer with similar underlying causes.
A team of researchers from the University of Tokyo and their collaborators focused on therapies targeting H3K27me3, a modification on a DNA-packaging histone protein, which plays a large role in regulating gene expression. The modification occurs when methyl groups, each consisting of three hydrogen atoms bonded to a single carbon atom (CH3), are added to the protein in a process called methylation.
The modification, also referred to as being epigenetic (a heritable change in gene function that occurs without altering the sequence of the DNA), has been tied to the repression, or reducing the expression, of tumor suppressor genes, with the accumulation of the methylated histones around the genes.
Because of its effects on repressing genes, H3K27me3 is being targeted by therapies to correct some of the disordered gene expression observed in cancer cells. While this therapy is effective for some cancers, the mechanism of H3K27me3 therapies on tumor cells was not yet known.
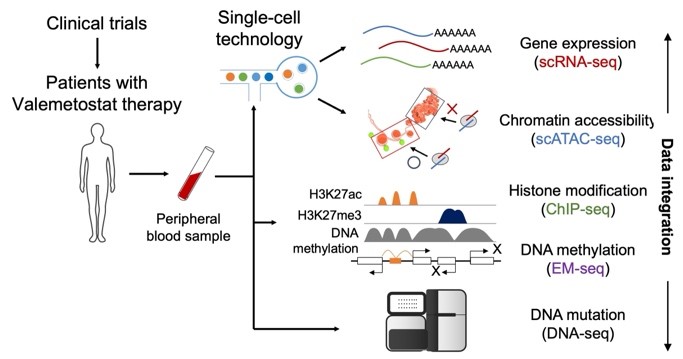
Research workflow
Researchers used single-cell analysis of tumor cells to study the mechanism and efficacy of valemetostat in clinical trials of patients undergoing therapy with the cancer drug. ©2024 Makoto Yamagishi, The University of Tokyo
The research team conducted a study characterizing the effects of H3K27me3 on cancer cells by treating patients who have adult T-cell leukemia/lymphoma (ATL), a rare type of blood cancer, with valemetostat. The drug prevents the methylation of histone H3 by inhibiting the histone-modifying enzymes EZH1 and EZH2, which increase H3K27 and have been found to be abnormal in cancers. Treating patients with valemetostat decreased H3K27me3 and the condensation of DNA, opening up several tumor-suppressor genes for expression in cancer cells.
“Before H3K27me3-inhibitor therapies were developed, no effective treatments for blood cancers with accumulated genetic abnormalities existed, and new treatments needed to be developed,” said Makoto Yamagishi, first author of the paper and associate professor at the Graduate School of Frontier Sciences at the University of Tokyo. “Once we established that these therapies were effective against some types of cancer, understanding the therapeutic mechanisms of these drugs became extremely important.”
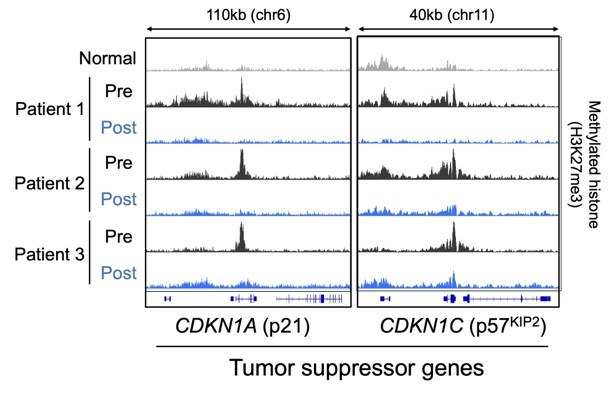
Reactivation of tumor suppressor genes by valemetostat
In patients who receive treatment, methylated histones (H3K27me3) that have accumulated around many tumor suppressor genes disappear and their expression is restored. ©2024 Makoto Yamagishi, The University of Tokyo
The team established that valemetostat treatment decreased tumor size and produced a durable clinical response to therapy in the clinical trial of patients with ATL, an aggressive cancer with many genetic mutations. The patients were able to safely remain on valemetostat treatment for more than two years.
“In blood cancers with poor prognosis due to genetic abnormalities, epigenetic mechanisms mediated by methylated histones can be targeted therapeutically,” said Professor Kaoru Uchimaru, also of the Graduate School of Frontier Sciences and last author of the study. “Valemetostat can restore expression of many tumor suppressor genes and sustainably inhibit tumor cell growth.”
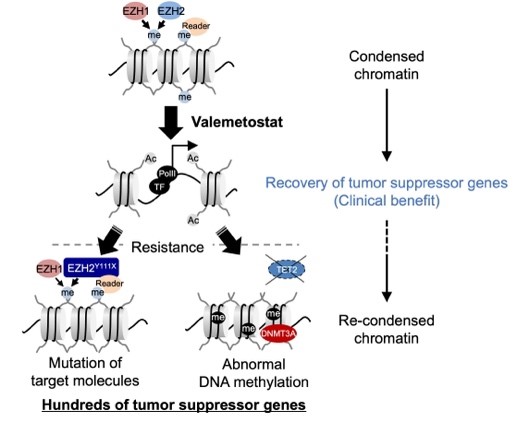
Mechanisms of action and resistance in histone methylation-targeted therapy
Valemetostat reduces methylated histone levels by inhibiting EZH1/2 and induces expression of tumor suppressor genes, resulting in a sustained clinical benefit. In some patients receiving long-term therapy, mutations in target molecules or aberrant DNA methylation can cause chromatin structure reaggregation, leading to clinical relapse. ©2024 Makoto Yamagishi, The University of Tokyo
Characterizing the mechanism of valemetostat therapy in patients with ATL is a huge step forward for epigenetic cancer treatments that target the expression of genes in cancer cells. The team recognizes, however, that many challenges remain. Cancers can become resistant to H3K27me3-inhibitor therapies if patients are treated for long periods of time, allowing cancer to recur. In some cases, cancer cells had acquired new mutations that interfered with valemetostat’s effectiveness over time and reduced long-term patient response to the drug.
“EZH1/2 inhibitors are effective treatments, but a mechanism of resistance to long-term treatment has also been identified,” said Yamagishi. “Based on this mechanism of resistance, it is important to continue to improve treatment methods and develop combination therapies that provide longer-term therapeutic effects.”








