A team of researchers has made significant strides in understanding metal-nitrogen-carbon (M-N-C) catalysts, offering alternatives to expensive platinum-group-metal (PGM) catalysts and a pathway to a greener future.
Details of their findings were published in the Journal of Materials Chemistry A on May 1, 2024.
Hydrogen, known as the “fuel of the future,” offers numerous advantages in the transition towards a low-carbon economy. Its versatility allows for applications across multiple sectors, including transportation, where hydrogen fuel cells can power vehicles, thereby reducing greenhouse gas emissions and mitigating climate change. However, significant challenges persist in oxygen electrocatalysis, hindering the development of large-scale techniques for hydrogen generation and usage based on green electricity.
One of the longstanding challenges is the reliance on costly PGM catalysts to drive oxygen electrocatalysis. In response to these challenges, researchers have turned to M-N-C catalysts as a promising alternative. Reports over the past decade have shown that M-N-C catalysts, doped with earth-abundant metal elements like 3d metals, offer versatile performance in oxygen electrocatalysis, some comparable to PGM catalysts. Yet the exact mechanics behind their electrocatalytic activities remains lacking; key factors such as the potential of zero charge (PZC) and solvation effects have been overlooked in previous studies.
“We’re at a critical point in sustainable energy technologies,” says Di Zhang, an assistant professor at the Advanced Institute for Materials Research at Tohoku University and co-author of the paper. “Understanding the factors influencing M-N-C catalysts’ performance is essential for innovation in hydrogen generation and usage.”
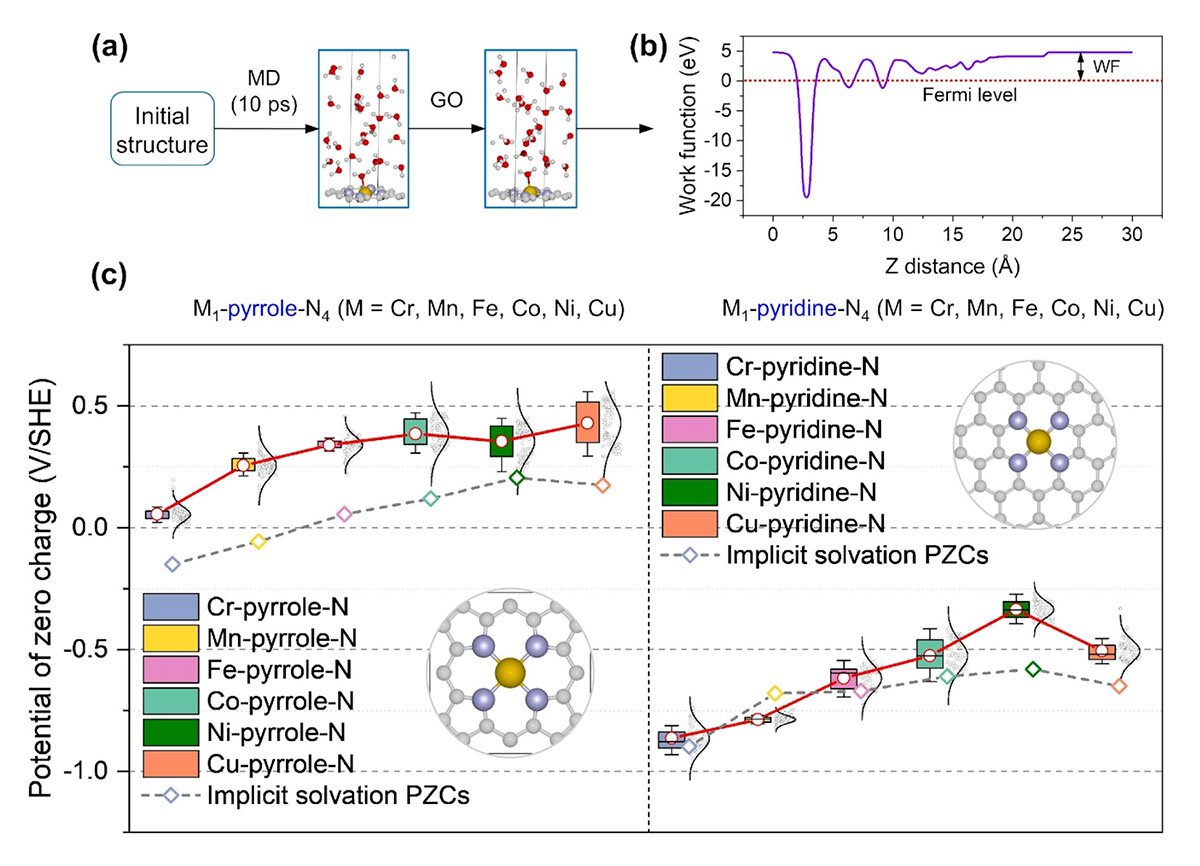
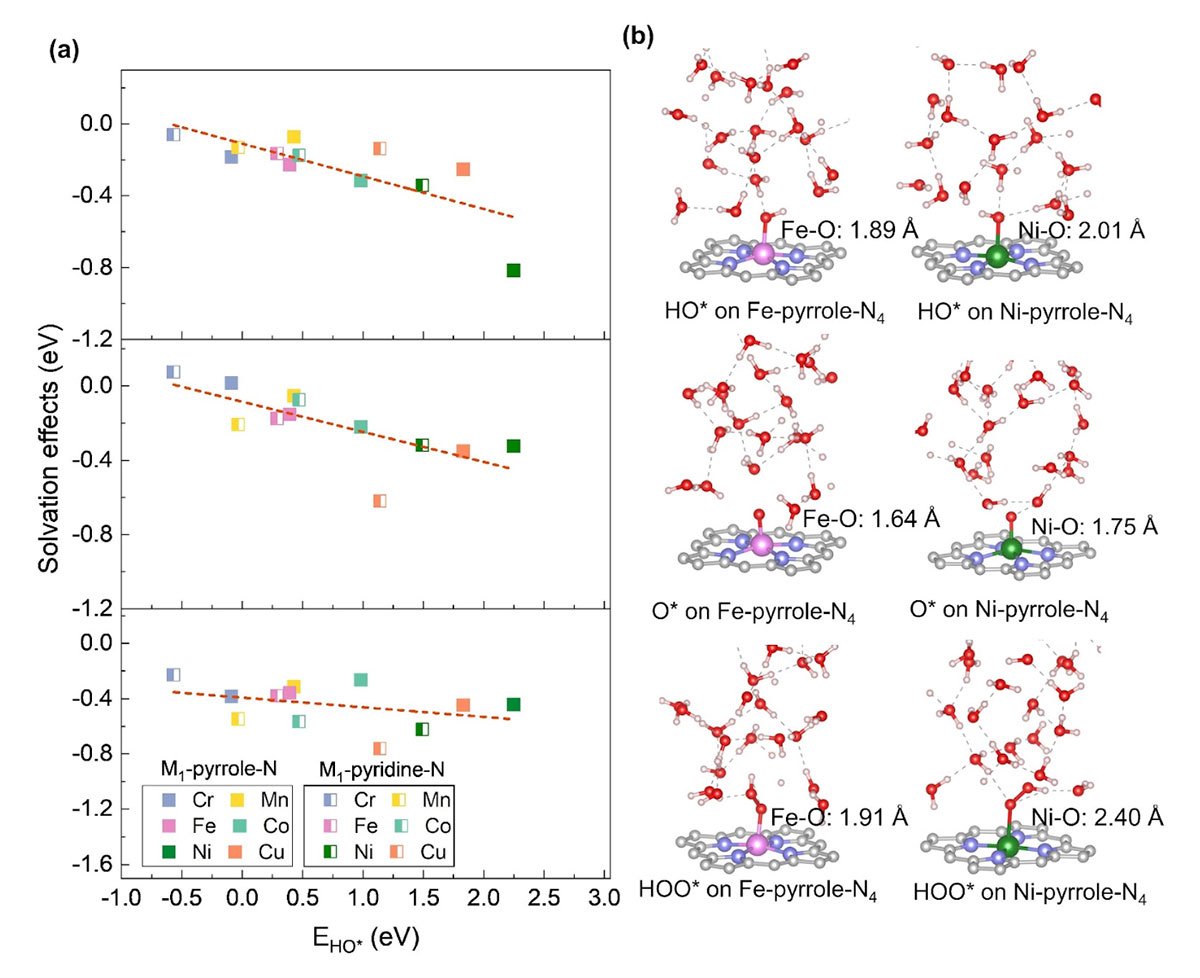
Zhang and his colleagues revealed that PZCs and solvation effects play a pivotal role in pH-dependent activities, significantly impacting reaction energetics. By conducting large scale sampling via ab initio molecular dynamics and density functional theory calculations, the researchers analyzed twelve distinct M-N-C configurations with explicit solvation models. They observed substantial variations in PZCs and solvation effects based on catalyst structures, metal types, and nitrogen configurations.
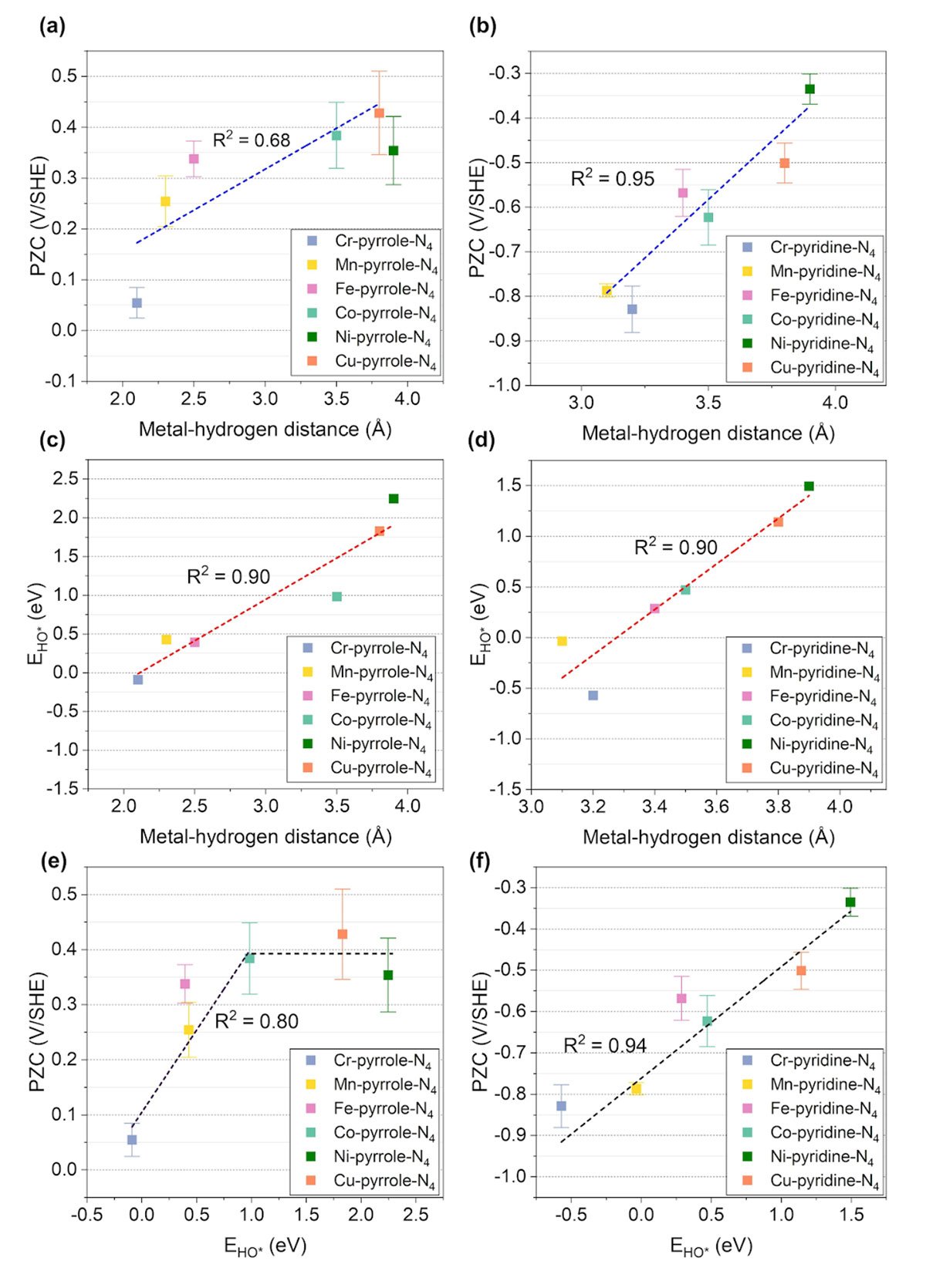
“Our findings underscore the importance of considering PZC and solvation effects in microkinetic modeling,” adds Zhang. “This knowledge is crucial for the rational design of high-performance M-N-C catalysts, accelerating the development of sustainable hydrogen technologies.”
- Publication Details:
Title: The Potential of Zero Charge and Solvation Effects on Single-Atom M-N-C Catalysts for Oxygen Electrocatalysis
Authors: Di Zhang and Hao Li
Journal: Journal of Materials Chemistry A
DOI:






