 PhD candidate Brenton Cook and Professor Dougal McCulloch in the RMIT Microscopy and Microanalysis Facility
PhD candidate Brenton Cook and Professor Dougal McCulloch in the RMIT Microscopy and Microanalysis Facility
An international team has made diamonds in minutes in a laboratory at room temperature – a process that normally takes billions of years, huge amounts of pressure and super-hot temperatures.
The team, led by RMIT University and The Australian ³Ô¹ÏÍøÕ¾ University (ANU), working with the University of Sydney and Oak Ridge ³Ô¹ÏÍøÕ¾ Laboratory in the United States, made two types of diamonds: the kind found on an engagement ring and another type of diamond called Lonsdaleite, which is found in nature at the site of meteorite impacts such as Canyon Diablo in the US.
One of the lead researchers, Professor Dougal McCulloch and his team at RMIT used advanced electron microscopy techniques to capture solid and intact slices from the experimental samples to create snapshots of how the two types of diamond formed.
“Our pictures showed that the regular diamonds only form in the middle of these Lonsdaleite veins under this new method developed by our cross-institutional team,” McCulloch said.
“Seeing these little ‘rivers’ of Lonsdaleite and regular diamond for the first time was just amazing and really helps us understand how they might form.”
 PhD candidate Brenton Cook and Professor Dougal McCulloch in the RMIT Microscopy and Microanalysis Facility
PhD candidate Brenton Cook and Professor Dougal McCulloch in the RMIT Microscopy and Microanalysis Facility
Lonsdaleite, named after the crystallographer Dame Kathleen Lonsdale, the first woman elected as a Fellow to the Royal Society, has a different crystal structure to regular diamond. It is predicted to be 58 per cent harder.
Co-lead researcher, Professor Jodie Bradby from the ANU Research School of Physics said the breakthrough shows that Superman may have had a similar trick up his sleeve , without using his heat ray.
“Natural diamonds are usually formed over billions of years, about 150 kilometres deep in the Earth where there are high pressures and temperatures above 1,000 degrees Celsius,” said Bradby.
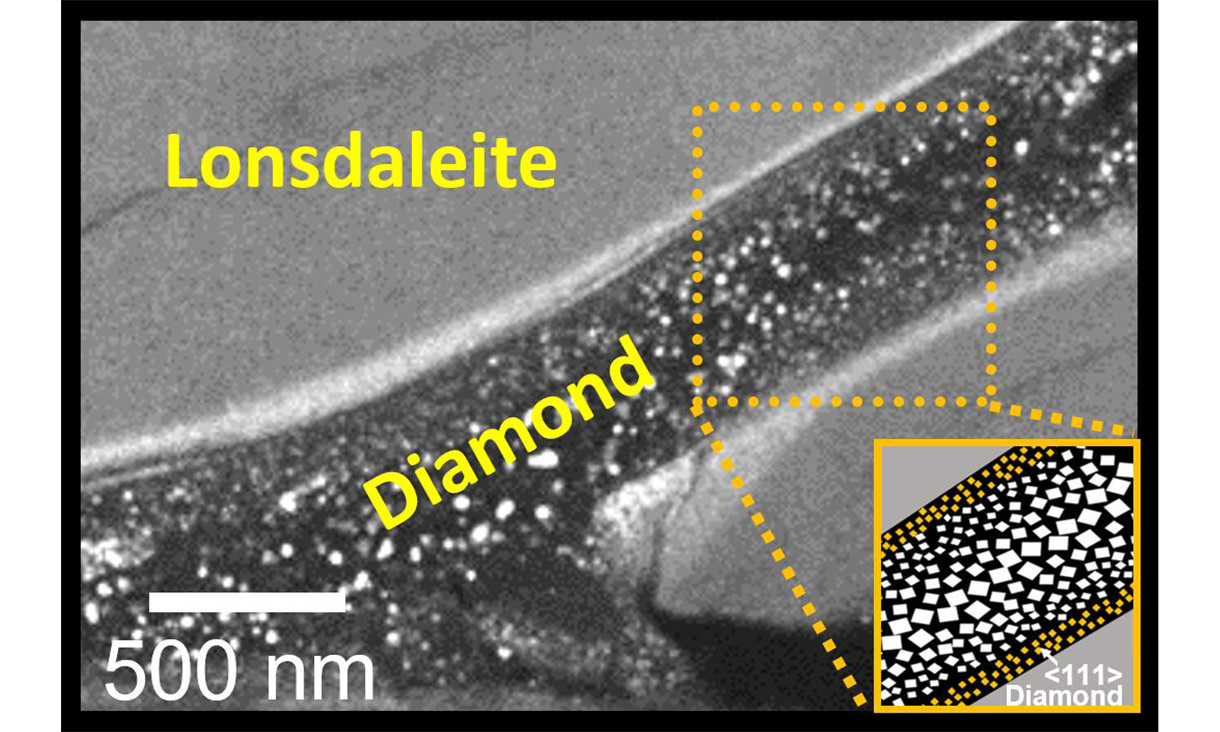 RMIT researchers captured ‘rivers’ of Lonsdaleite and regular diamond.
RMIT researchers captured ‘rivers’ of Lonsdaleite and regular diamond.
The team previously created Lonsdaleite in the lab only at high temperatures.
But this new unexpected discovery shows both Lonsdaleite and regular diamond can also form at normal room temperatures by just applying high pressures of 100 GPa – equivalent to 640 African elephants on the tip of a ballet shoe.
“The twist in the story is how we apply the pressure. As well as very high pressures, we allow the carbon to also experience something called ‘shear’ – which is like a twisting or sliding force. We think this allows the carbon atoms to move into place and form Lonsdaleite and regular diamond,” Bradby said.
“Lonsdaleite has the potential to be used for cutting through ultra-solid materials on mining sites,” Bradby said.
“Creating more of this rare but super useful diamond is the long-term aim of this work.”
The researchers acknowledge the support of the .
‘‘, with collaborators from The Australian ³Ô¹ÏÍøÕ¾ University, University of Sydney and Oak Ridge ³Ô¹ÏÍøÕ¾ Laboratory, is published in Small (DOI: 10.1002/smll.202004695).







