Lanthanum trihydride, a compound of lanthanum and hydrogen, when lightly doped with oxygen shows potential as an efficient hydrogen carrier, according to a new study by Tokyo Tech researchers. Hydride ion (H–) conductors are expected to be used in chemical reactors and energy storage systems. However, the low H– conductivity at room temperature introduces certain technical limitations. These limitations may now be overcome with this latest innovation by the researchers.
Fossil fuels such as coal, oil, and natural gas cannot last forever. Therefore, gradually decreasing our dependency on fossil fuels seems critical. Whereas alternative sources of energy such as solar or tidal energy can fill in the gap to some extent, they come with certain practical limitations. For example, utilizing solar energy requires the use of solar panels with large surface areas, thus making it a relatively expensive energy alternative.
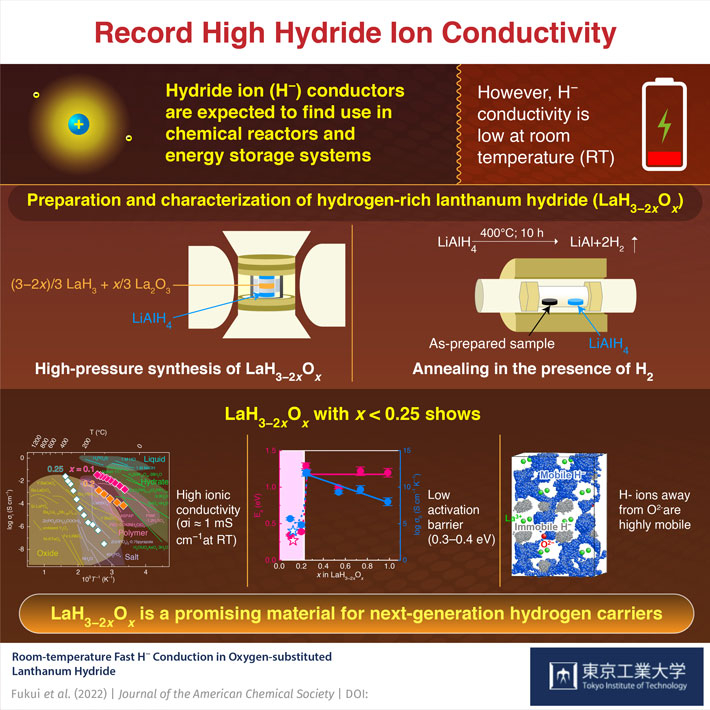
In the recent past, scientists have explored multiple possibilities in an attempt to harness energy from various other sources. One such example includes the use of hydrogen-based energy systems. In this regard, lanthanum hydride, a compound of hydrogen and the metallic element lanthanum, has attracted quite a lot of attention. Because of its unique material properties, lanthanum hydride allows for superior hydride ion (H–) conductivity under certain conditions, which is the prerequisite for the efficient operation of chemical reactors and energy storage systems. However, most H– conductors show low H– conductivity at room temperature, which limits their application.
In a new study, researchers from Tokyo Institute of Technology (Tokyo Tech) have now come up with a technological innovation that can be used to overcome this limitation and design the next generation of energy carriers. The research team, led by Prof. Hideo Hosono, senior author of the study and Honorary Professor, Tokyo Tech, has successfully prepared and characterized a hydrogen-rich lanthanum hydride, with the chemical formula “LaH3−2xOx,” which shows a H– conductivity that is higher by three orders of magnitude when compared with the best conductor available. Their trick was to control the concentration of oxygen in LaH3−2xOx.
The researchers used a two-step process to prepare LaH3−2xOx. The high-density LaH3−2xOx pellet prepared using the first high-pressure synthesis step had a large amount of hydrogen deficiency. Next, the researchers exposed these pellets to hydrogen gas atmosphere at an elevated temperature (400 °C) for an extended duration (10 hours) to fill the hydrogen vacancy. It resulted in the formation of “LaH2.8O0.1,” a novel material showing high ionic conductivity even at room temperature.
Elaborating the concept behind their research, which is all set to be published in the Journal of the American Chemical Society, Prof. Hosono says, “Our study was driven by the idea that minimizing the amount of substituted O2− used to suppress the electronic conduction in LaH3−y should ideally make fast H− conduction in LaH3−2xOx at room temperature possible.”
Quite interestingly, the hydrogen-rich LaH3−2xOx also exhibited a low activation barrier—an energy hurdle that it must overcome to successfully function as an efficient ionic conductor. In terms of the actual measurement, this low activation barrier was somewhere between 0.3 and 0.4 eV. Moreover, the low activation barrier was independently confirmed using computerized simulations. The simulations also showed that the H– ions far from O2− ions were highly mobile and some of them traveled long distances by knocking each other out, suggesting the presence of strong repulsive Coulombic interactions ideal for fast H– conduction.
It seems there is good reason for Prof. Hosono to observe optimistically, “Hydrogen-rich LaH3−2xOx is a promising candidate for next-generation hydrogen carriers and can promote fossil fuel replacement!”
The world sure has its fingers crossed as it (energetically) waits for a fossil-fuel-free future!
Reference
Authors : | Keiga Fukui1, Soshi Iimura1,2,3, Albert Iskandarov4, Tomofumi Tada1,5, and Hideo Hosono1,3 |
Title of original paper : | Room-temperature fast H– conduction in oxygen-substituted lanthanum hydride |
Journal : | Journal of the American Chemical Society |
DOI : | |
Affiliations : | 1 Materials Research Center for Element Strategy, Tokyo Institute of Technology, Yokohama 226-8503, Japan 2 PRESTO, Japan Science and Technology Agency, Kawaguchi, Saitama 332-0012, Japan 3 ³Ô¹ÏÍøÕ¾ Institute for Materials Science (NIMS), Tsukuba, Ibaraki 305-0047, Japan 4 Graduate School of Nanobioscience, Yokohama City University, Yokohama 236-0027, Japan 5 Kyushu University Platform of Inter/Transdisciplinary Energy Research, Kyushu University, Fukuoka 819-0395, Japan |








