Observing cell proliferation in living animals for a long period requires collecting and analyzing animal organs at multiple points in time. This cumbersome process requires an abundance of resources, including animals.
Now, a research group has developed a genetically modified mouse where the proliferation of intended cells in the body can be monitored over time, and with high sensitivity. This simple and cost-effective monitoring method requires only a small amount of blood to be drawn.
“Our genetically modified mouse produces a protein called luciferase and releases it into the blood,” points out Associate Professor Junta Imai from the Department of Metabolism and Diabetes within Tohoku University’s Graduate School of Medicine. “Luciferase is released into the bloodstream when the targeted cells undergo proliferation and we could collect small blood samples from the mice to measure luciferase levels, enabling real-time detection of cell proliferation within the body.”
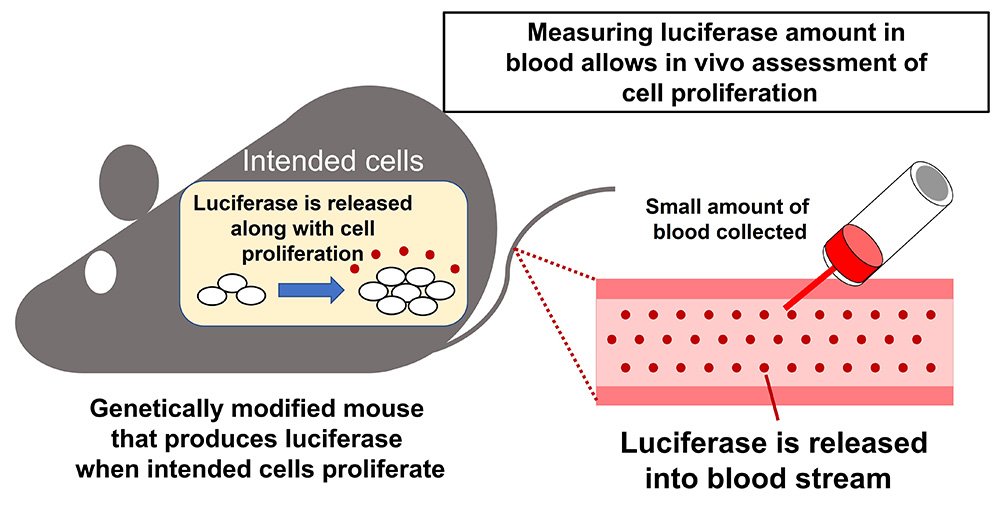
This innovative method can also be genetically modified to observe the proliferation of various types of intended cells.
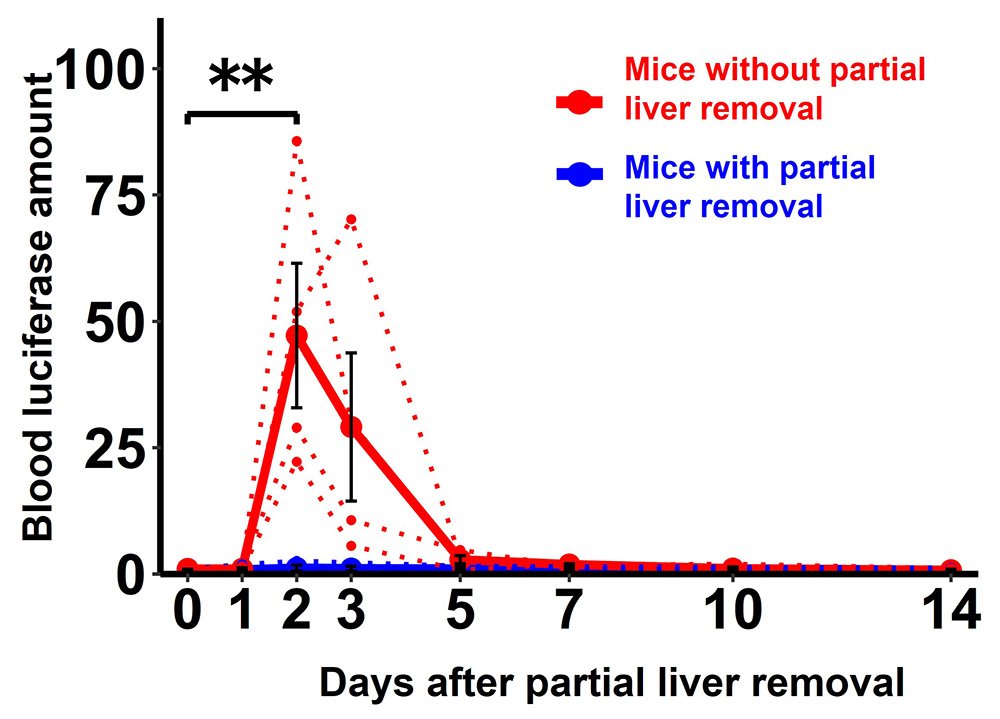
First, Imai and his colleagues, including Assistant Professor Hiroto Sugawara and Professor Hideki Katagiri of the Tohoku University Graduate School of Medicine, subjected the mice to monitoring for the proliferation of liver cells.
When the liver is partially removed, the remaining liver cells reportedly proliferate. Therefore, blood samples were taken from the mice after partial removal of the liver and further monitored for proliferation of liver cells. This approach allows changes in liver cell proliferation to be monitored with very high sensitivity.
Next, the research group monitored the proliferation of pancreatic β cells, insulin-producing cells that occupy the body in very small numbers. The group’s previous study showed that neural signals cause β-cell proliferation. Putting this into practice, the group stimulated β-cell proliferation in the mice with neural signals and then observed over time. Changes in β-cell proliferation were thereby monitored with high sensitivity.
These results indicate that the method can detect the proliferation of even small cell populations. Furthermore, cells from these mice were found to potentially be applicable in the search for agents that increase cell proliferation.
“These methods are highly anticipated to facilitate the development of treatments for many diseases, such as curative agents for diabetes that increase insulin-producing cells and drugs that inhibit the growth of cancer cells, while making optimally effective use of limited experimental resources,” adds Imai.
The study was published in the international journal Nature Communications and was supported by the Japan Science and Technology Agency, JST, [Moonshot R&D] as well as by the Japan Agency for Medical Research and Development, AMED (AMED-PRIME).

- Publication Details:
Title: A highly sensitive strategy for monitoring real-time proliferation of targeted cell types in vivo
Authors: Hiroto Sugawara, Junta Imai, Junpei Yamamoto, Tomohito Izumi, Yohei Kawana, Akira Endo, Masato Kohata, Junro Seike, Haremaru Kubo, Hiroshi Komamura, Yuichiro Munakata, Yoichiro Asai, Shinichiro Hosaka, Shojiro Sawada, Shinjiro Kodama, Kei Takahashi, Keizo Kaneko, and Hideki Katagiri
Journal: Nature Communications
DOI: