Aquaporin (Aqp) 10 water channels in humans allow the free passage of water, glycerol, urea, and boric acid across cells. However, Aqp10.2b in pufferfishes allows only the passage of water and glycerol and not urea and boric acid. Researchers from the Tokyo Institute of Technology sought to understand the evolutionary timeline that resulted in the variable substrate selection mechanisms among Aqp10s. Their results indicate that Aqp10.2 in ray-finned fishes may have reduced or lost urea and boric acid permeabilities through evolution.
Aquaporins (Aqps) are proteins that form water channels in the membranes of living cells, including those of bacteria, fungi, animals, and plants. These channels facilitate water transportation across cells more rapidly than diffusion through the membrane phospholipid bilayer.
Aqp10 belongs to the aquaglyceroporin subfamily of water channels. These proteins facilitate many of our body’s physiological processes, including gut function, liver and fat cell metabolism, and skin elasticity. Water and solutes, such as glycerol, urea, and boric acid, get transported through human Aqp10 depending on concentration gradients across the membrane.
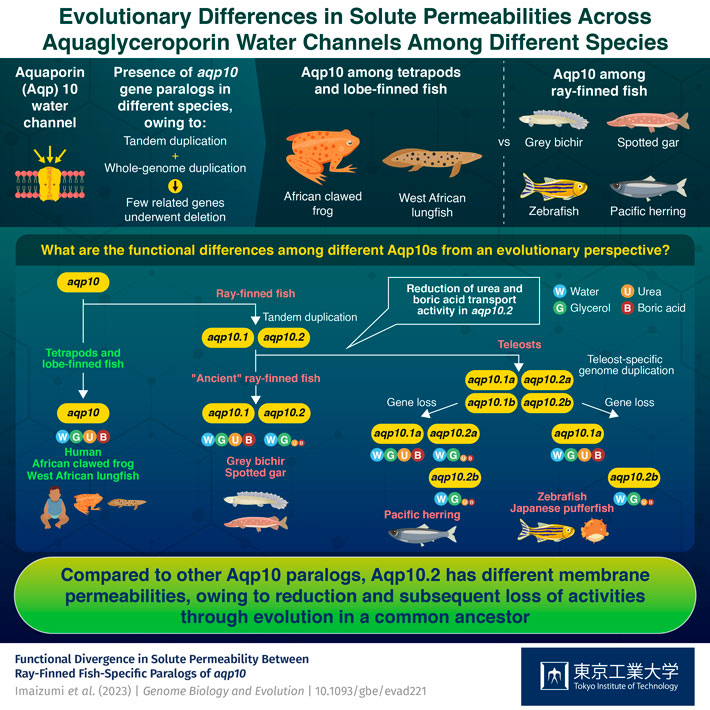
Sarcopterygians, which include coelacanths, lungfish, and tetrapods (such as amphibians, reptiles, birds, and mammals), are known to have a single gene that codes for Aqp10. In contrast, actinopterygians, such as ray-finned fishes, have paralogs, or near-identical copies, of the aqp10 gene, such as aqp10.1 and aqp10.2. Interestingly, the ray-finned Japanese pufferfish has paralog called aqp10.2b that shows permeability to water and glycerol but not to urea and boric acid.
To understand how these functional differences between solute permeabilities in Aqp10 in humans and pufferfish may have evolved, a team of researchers from the School of Life Science and Technology, Tokyo Institute of Technology (Tokyo Tech), Department of Advanced Bioscience, Faculty of Agriculture, Kindai University, Department of Integrative Biology at Michigan State University, Aquamarine Fukushima AMF, and Department of Physiology and Biomedical Engineering at the Mayo Clinic College of Medicine and Science analyzed Aqp10s in different species.
When asked about their study, Dr. Ayumi Nagashima, who is an Assistant Professor in the Department of Life Science and Technology at Tokyo Tech and the lead scientist of the study, explains, “Evolutionary adaptations in solute selectivity could present a promising model for analyzing solute permeability evolution in aquaglyceroporins. In this study, to elucidate the evolutionary history of Aqp10 solute selectivity, we analyzed and compared the permeability and evolutionary relationships of Aqp10s in eight bony vertebrate species.”
The research team found that similar to the tetrapod and lobe-finned fish Aqp10s, Aqp10.1 in ray-finned fishes also transport water, glycerol, urea, and boric acid. On the other hand, while Aqp10.2 in ray-finned fishes transport water and glycerol, they restrict urea and boric acid passage much more than Aqp10 and Aqp10.1.
These intriguing results indicate that water, glycerol, urea, and boric acid permeabilities are plesiomorphic features of Aqp10 water channels in all tetrapods and lobe-finned fish. However, the Aqp10.2 found in ray-finned fish may have reduced or lost urea and boric acid permeability during evolution.
In this regard, Dr. Nagashima remarks, “Our study showed that the Aqp10.2 of ray-finned fishes allows only limited or no urea or boric acid transport across it. These activities were likely systematically reduced and subsequently lost through evolution in the common ancestor of these fishes. This research is expected to shed light on the elucidation of the substrate selection mechanism of aquaglyceroporins, which contributes to nutrient transport and other processes in the future.”
In summary, this study highlights that water, glycerol, urea, and boric acid transport activities are plesiomorphic activities of Aqp10 characteristic of the ancestral type common to the studied species. Consequently, the results suggest that Aqp10.2 in actinopterygians evolved to diminish the transport activity of urea and boric acid.
Reference
Authors : | Genki Imaizumi1, Kazutaka Ushio1, Hidenori Nishihara1,2, Ingo Braasch3, Erika Watanabe1, Shiori Kumagai1, Tadaomi Furuta1, Koji Matsuzaki4, Michael Romero5,6, Akira Kato1 and Ayumi Nagashima1 |
Title : | Functional divergence in solute permeability between ray-finned fish-specific paralogs of aqp10 |
Journal : | Genome Biology and Evolution |
DOI : | |
Affiliations : | 1 School of Life Science and Technology, Tokyo Institute of Technology, Japan 2 Department of Advanced Bioscience, Faculty of Agriculture, Kindai University, Japan 3 Department of Integrative Biology, College of Natural Science, Michigan State University, USA 4 Marine Science Museum (Aquamarine Fukushima AMF), Japan 5 Department of Physiology and Biomedical Engineering, Mayo Clinic College of Medicine and Science, USA 6 Nephrology and Hypertension, Mayo Clinic College of Medicine and Science, USA |








