 Javier Rangel-Moreno and Shabaana Khader
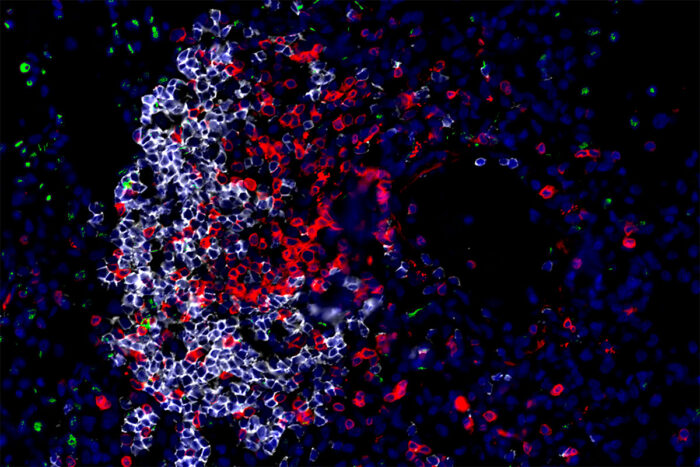
Javier Rangel-Moreno and Shabaana KhaderInnate (green) and adaptive (red and white) immune cells in a lung surround and kill the bacteria that cause tuberculosis. Researchers at Washington University School of Medicine in St. Louis and the Africa Health Research Institute have identified a master cell that coordinates the body’s immune defenses in the crucial early days after infection. Boosting the activity of such cells could help reduce the millions of new infections that occur worldwide every year.
In the first days after the tuberculosis (TB) bacteria infect the body, a flurry of immune cells are activated to fight the infection. Now, researchers have identified a master cell that coordinates the body’s immune defenses in those crucial early days, according to a new study from Washington University School of Medicine in St. Louis and Africa Health Research Institute in KwaZulu-Natal, South Africa.
The findings, published June 5 in the journal Nature, suggest that bolstering such cells’ activity could help prevent the deadly bacteria from gaining a foothold in the lungs and reduce the tens of millions of new infections that occur every year.
“The immune response to the TB bacteria hinges on the early response of this cell, and that opens up a whole new avenue for TB control,” said co-senior author , a professor and interim head of the at the School of Medicine. “Now we can start thinking about ways to target this cell to help the body fight off the bacteria before they get a chance to establish themselves.”
According to the World Health Organization, about 1.5 million people died of TB in 2017, making it the most lethal infectious disease worldwide. While a vaccine is available, it only provides good protection against the more severe forms of the disease in young children and is less effective in older children and adults. Despite being widely used, the vaccine has failed to halt transmission of the disease, and a quarter of the world’s population is infected with the TB bacteria.
“Positive results from several recent vaccine trials make this an exciting time to be working in TB immunology,” said co-senior author Alasdair Leslie, PhD, a faculty member at the Africa Health Research Institute. “The more we can understand about the interaction between the bacteria that cause TB and people, the more chance we have of building on these gains and defeating this deadly epidemic.”
Vaccines are designed to warn the immune system about dangerous microbes by presenting bits of such microbes to adaptive immune cells. These cells remember what they’ve seen and respond rapidly if and when such microbes show up – ideally, before the microbes multiply and cause disease. But in the case of TB, adaptive immunity alone, even when primed by vaccination, may be to protect people.
Khader, Leslie and colleagues – including co-first authors Amanda Ardain, a graduate student in Leslie’s lab, and Racquel Domingo-Gonzalez, PhD, and Shibali Das, PhD, both postdoctoral researchers in Khader’s lab – studied animals and people to identify the immune cells and proteins that defend the body against the TB bacteria in the first days after infection.
They found that cells known as group 3 innate lymphoid cells (ILC3) play a pivotal role in the first two weeks of infection. ILC3 cells belong to the innate branch of the immune system that detects and responds to foreign invaders in the body. Biologists have long believed that the innate immune system lacks memory for specific microbes, but recent studies suggest that some innate immune cells may have memory.
Experiments showed that within five days after infection, ILC3 cells show up in the lungs, where they release chemical compounds that activate and attract other immune cells. The arriving cells include other innate immune cells – which come loaded with bacteria-killing weapons – as well as adaptive immune cells that direct and enhance the innate immune cells’ killing potential. Together, the immune cells surround the bacteria and destroy them.
In mice that lack ILC3 cells, the immune responses are delayed and struggle to get off the ground. The activating chemical compounds are released later, immune cells are slower to arrive at the lungs, the bacteria are not engulfed by immune cells, and consequently, the mice are sicker and have more TB bacteria in the lungs. When the researchers gave ILC3 cells to mice that lacked their own ILC3 cells, it jump-started the immune response, and the bacterial numbers never rose very high.
“These innate lymphoid cells seem to orchestrate all the early downstream immune responses – both innate and adaptive – that you need to control infection,” said Khader, who is also a professor of pathology and immunology.
In people and animals sick with TB disease, ILC3 cells congregated in the lungs, especially in immune structures that surrounded and killed bacteria. After people were successfully treated with antibiotics, ILC3 cells became more plentiful in their bloodstream, suggesting that the cells were no longer needed in the lung to fight infection.
Vaccine developers have largely ignored the innate immune system since it is thought to lack the ability to remember specific microbes. But recent studies have shown that innate immune cells may have memory or can be trained to be more effective, thereby fortifying the body’s innate immune defenses and providing wide-ranging protection. The vaccine against TB – known as the BCG vaccine – was developed a century ago and designed to target the adaptive immune system. But it is now thought to work partly by training the innate immune system.
“Children who get the BCG vaccine are protected not only against TB but many different infectious diseases and cancer for a few years,” Khader said. “They have lower rates of sickness and death from all causes than children who weren’t vaccinated. We wouldn’t want to replace the BCG vaccine, but we may be able to find a compound that we can use to boost immunity in vaccinated children, when the effects of the BCG start to wear off.”
Khader’s group has begun screening a set of chemical compounds, looking for ones that enhance ILC3 activity and drive a stronger immune response in the first days after infection.
“It’s still an open question whether ILCs in the lung are trainable or have memory and how long the training or memory would last,” Khader said. “But if we can train them and get a population of these cells primed and ready to go in the lung, that might be one way we can make a more effective vaccine for TB.”
Leslie added, “This study was an outstanding collaboration between scientists in South Africa and the U.S., and perfectly illustrates the power of bringing together expertise across continents to advance TB science.”





