The unique quantum properties of bismuth selenide make it a promising catalyst for the synthesis of organic ureas, as demonstrated by scientists at Tokyo Tech. Thanks to its topological surface states, the proposed catalyst exhibits remarkably high catalytic activity and durability when used for the synthesis of various urea derivatives, which are widely utilized as nitrogen fertilizers.
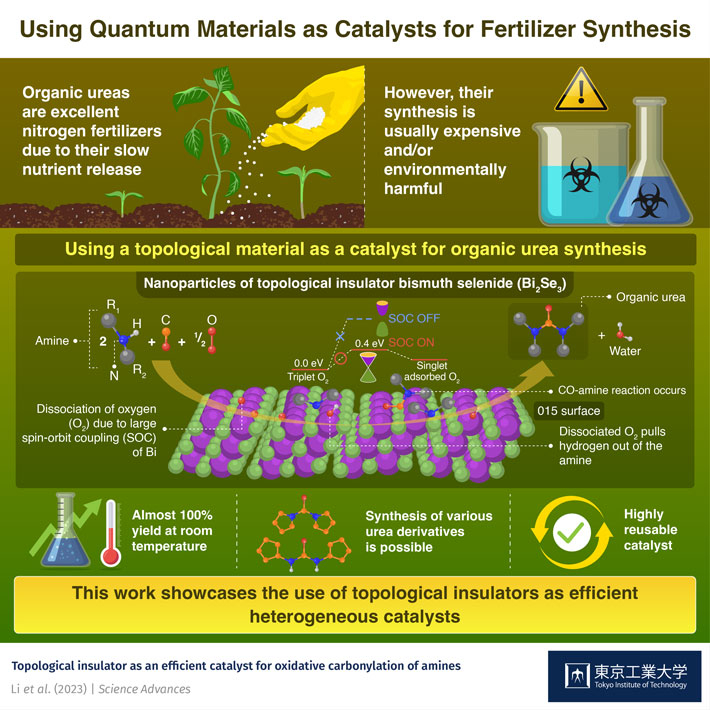
Synthetic fertilizers, one the most important developments in modern agriculture, have enabled many countries to secure a stable food supply. Among them, organic ureas (or organoureas) have become prominent sources of nitrogen for crops. Since these compounds do not dissolve immediately in water, but instead are slowly decomposed by soil microorganisms, they provide a stable and controlled supply of nitrogen, which is crucial for plant growth and function.
However, traditional methods to synthesize organoureas are environmentally harmful due to their use of toxic substances, such as phosgene. Although alternative synthesis strategies have been demonstrated, these either rely on expensive and scarce noble metals or employ catalysts that cannot be reused easily.
In a recent effort to address these challenges, a research team including Honorary Professor Hideo Hosono from Tokyo Institute of Technology, Japan, has leveraged the quantum properties of bismuth selenide (Bi2Se3) to synthesize organoureas. Their study will be published online on September 23, 2023, in Science Advances, a sister journal of the prestigious US journal, Science.
The researchers sought to take advantage of the fact that Bi2Se3 is a topological insulator. Its extremely robust surface possesses unique electronic properties, making it a lucrative candidate for catalytic applications. Accordingly, the team prepared Bi2Se3 nanoparticles to maximize the surface area of the catalyst. They exploited its surface toughness and large spin–orbit interactions to produce various organic urea derivatives from two amine molecules, as well as carbon monoxide (CO) and oxygen (O2), achieving almost 100% yield at room temperature in many cases.
To shed light on the reaction mechanism, the team investigated the surface states of Bi2Se3 through molecular simulations. One of the key steps in the synthesis process was the removal of the hydrogen atom from the amines, so that they could be subsequently linked together by a –CO group. The researchers found that O2 molecules bind stably to Bi atoms on the (015) surface of Bi2Se3, which changes their spin state from triplet to singlet. This causes the O2 molecules to dissociate. The adsorbed dissociated oxygen groups pull hydrogen out of the amines bound to Se, giving way to the CO–amine reaction.
“We found that the spin state of the O2 molecule is changed from triplet to singlet by the local magnetic field arising from the strong spin–orbit interaction of Bi and the singlet O2 with much higher reactivity pulls hydrogen out from the amine, reducing energy barrier for the desired reaction,” explains Prof. Hosono. “This catalytic effect is a result of the unique features of topological materials and the appropriate element choice of Bi and Se for this reaction.”
The activity of the Bi2Se3 catalyst exceeded that of other Se-containing compounds, as well as that of most existing transition metal-based catalysts. The surface of the proposed catalyst nanoparticles was remarkably stable as well, thanks to its topological features. “The recyclability of a catalyst is one of its most critical properties for practical applications. The proposed Bi2Se3 catalyst could be reused at least 20 times without obvious loss of catalytic activity, whereas the yields for other Se-based catalysts decreased continuously,” highlights Prof. Hosono.
This groundbreaking work not only provides a viable solution to the challenges associated with urea synthesis, but also showcases the potential of leveraging specific quantum properties of materials for various novel applications, including sustainable agricultural practices.
Reference
Authors : | Jiang Li1, Jiazhen Wu1,2, Sang-won Park1,3, Masato Sasase1, Tian-Nan Ye1,4, Yangfan Lu1,5, Masayoshi Miyazaki1, Toshiharu Yokoyama1, Tomofumi Tada1,*, Masaaki Kitano1,*, and Hideo Hosono1,6,* |
Title : | Topological insulator as an efficient catalyst for oxidative carbonylation of amines |
Journal : | Science Advances |
DOI : | |
Affiliations : | 1 MDX Research Center for Element Strategy, International Research Frontiers Initiative, Tokyo Institute of Technology 2 Department of Materials Science and Engineering, Southern University of Science and Technology 3 Department of Chemical and Materials Engineering, University of Suwon 4 Frontiers Science Center for Transformative Molecules, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University 5 College of Materials Science and Engineering, ³Ô¹ÏÍøÕ¾ Engineering Research Center for Magnesium Alloys, Chongqing University 6 International Center for Materials Nanoarchitectonics (WPI-MANA), ³Ô¹ÏÍøÕ¾ Institute for Materials Science (NIMS) |








